A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel
- PMID: 19164516
- PMCID: PMC2629443
- DOI: 10.1073/pnas.0808584106
A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel
Abstract
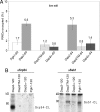
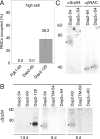
Sorting of eukaryotic membrane and secretory proteins depends on recognition of ribosome-bound nascent chain signal sequences by the signal recognition particle (SRP). The current model suggests that the SRP cycle is initiated when a signal sequence emerges from the ribosomal tunnel and binds to SRP. Then elongation is slowed until the SRP-bound ribosome-nascent chain complex (RNC) is targeted to the SRP receptor in the endoplasmic reticulum (ER) membrane. The RNC is then transferred to the translocon, SRP is released, and translation resumes. Because RNCs do not target to the translocon efficiently if nascent chains become too long, the window for SRP to identify its substrates is short. We now show that a transmembrane signal-anchor sequence (SA) significantly enhances binding of SRP to RNCs even before the SA emerges from the ribosomal tunnel. In this mode, SRP does not contact the SA directly but is in close proximity to the portion of the nascent polypeptide that has already left the ribosomal tunnel. Early recruitment of SRP provides a mechanism to expand the window for substrate identification. We suggest that the dynamics of the SRP-ribosome interaction is affected not only by the direct binding of SRP to an exposed signal sequence but also by properties of the translating ribosome that are triggered from within the tunnel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Martoglio B, Dobberstein B. Signal sequences: More than just greasy peptides. Trends Cell Biol. 1998;8:410–415. - PubMed
-
- Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70:755–775. - PubMed
-
- Shan SO, Walter P. Co-translational protein targeting by the signal recognition particle. FEBS Lett. 2005;579:921–926. - PubMed
-
- Halic M, Beckmann R. The signal recognition particle and its interactions during protein targeting. Curr Opin Struct Biol. 2005;15:116–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

