Disulfide bond formation by exported glutaredoxin indicates glutathione's presence in the E. coli periplasm
- PMID: 19164554
- PMCID: PMC2635786
- DOI: 10.1073/pnas.0812596106
Disulfide bond formation by exported glutaredoxin indicates glutathione's presence in the E. coli periplasm
Abstract
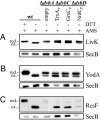
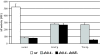
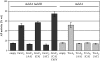
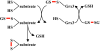
Organisms have evolved elaborate systems that ensure the homeostasis of the thiol redox environment in their intracellular compartments. In Escherichia coli, the cytoplasm is kept under reducing conditions by the thioredoxins with the help of thioredoxin reductase and the glutaredoxins with the small molecule glutathione and glutathione reductase. As a result, disulfide bonds are constantly resolved in this compartment. In contrast to the cytoplasm, the periplasm of E. coli is maintained in an oxidized state by DsbA, which is recycled by DsbB. Thioredoxin 1, when exported to the periplasm turns from a disulfide bond reductase to an oxidase that, like DsbA, is dependent on DsbB. In this study we set out to investigate whether a subclass of the thioredoxin superfamily, the glutaredoxins, can become disulfide bond-formation catalysts when they are exported to the periplasm. We find that glutaredoxins can promote disulfide bond formation in the periplasm. However, contrary to the behavior of thioredoxin 1 in this environment, the glutaredoxins do so independently of DsbB. Furthermore, we show that glutaredoxin 3 requires the glutathione biosynthesis pathway for its function and can oxidize substrates with only a single active-site cysteine. Our data provides in vivo evidence suggesting that oxidized glutathione is present in the E. coli periplasm in biologically significant concentrations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The reductive enzyme thioredoxin 1 acts as an oxidant when it is exported to the Escherichia coli periplasm.Proc Natl Acad Sci U S A. 1998 Sep 1;95(18):10751-6. doi: 10.1073/pnas.95.18.10751. Proc Natl Acad Sci U S A. 1998. PMID: 9724776 Free PMC article.
-
The role of glutathione in periplasmic redox homeostasis and oxidative protein folding in Escherichia coli.Redox Biol. 2023 Aug;64:102800. doi: 10.1016/j.redox.2023.102800. Epub 2023 Jun 26. Redox Biol. 2023. PMID: 37413765 Free PMC article.
-
The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm.J Biol Chem. 1997 Jun 20;272(25):15661-7. doi: 10.1074/jbc.272.25.15661. J Biol Chem. 1997. PMID: 9188456
-
Disulfide bond formation system in Escherichia coli.J Biochem. 2009 Nov;146(5):591-7. doi: 10.1093/jb/mvp102. Epub 2009 Jun 29. J Biochem. 2009. PMID: 19567379 Review.
-
Roles of thiol-redox pathways in bacteria.Annu Rev Microbiol. 2001;55:21-48. doi: 10.1146/annurev.micro.55.1.21. Annu Rev Microbiol. 2001. PMID: 11544348 Review.
Cited by
-
Lumen Thiol Oxidoreductase1, a disulfide bond-forming catalyst, is required for the assembly of photosystem II in Arabidopsis.Plant Cell. 2011 Dec;23(12):4462-75. doi: 10.1105/tpc.111.089680. Epub 2011 Dec 30. Plant Cell. 2011. PMID: 22209765 Free PMC article.
-
Interplay between drug efflux and antioxidants in Escherichia coli resistance to antibiotics.Antimicrob Agents Chemother. 2010 Dec;54(12):5366-8. doi: 10.1128/AAC.00719-10. Epub 2010 Sep 27. Antimicrob Agents Chemother. 2010. PMID: 20876376 Free PMC article.
-
TrbB from conjugative plasmid F is a structurally distinct disulfide isomerase that requires DsbD for redox state maintenance.J Bacteriol. 2011 Sep;193(18):4588-97. doi: 10.1128/JB.00351-11. Epub 2011 Jul 8. J Bacteriol. 2011. PMID: 21742866 Free PMC article.
-
Tellurite and Selenite: how can these two oxyanions be chemically different yet so similar in the way they are transformed to their metal forms by bacteria?Biol Res. 2022 Apr 5;55(1):17. doi: 10.1186/s40659-022-00378-2. Biol Res. 2022. PMID: 35382884 Free PMC article. Review.
-
Mechanisms of oxidative protein folding in the bacterial cell envelope.Antioxid Redox Signal. 2010 Oct;13(8):1231-46. doi: 10.1089/ars.2010.3187. Antioxid Redox Signal. 2010. PMID: 20367276 Free PMC article. Review.
References
-
- Aslund F, Berndt KD, Holmgren A. Redox potentials of glutaredoxins and other thiol-disulfide oxidoreductases of the thioredoxin superfamily determined by direct protein–protein redox equilibria. J Biol Chem. 1997;272:30780–30786. - PubMed
-
- Masip L, et al. An engineered pathway for the formation of protein disulfide bonds. Science. 2004;303:1185–1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

