Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery
- PMID: 19164788
- PMCID: PMC3718309
- DOI: 10.1161/STROKEAHA.108.531236
Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery
Abstract
Background and purpose: Cognitive decline after cardiac surgery remains common and diminishes patients' quality of life. Based on experimental and clinical evidence, this study assessed the potential of intravenously administered lidocaine to reduce postoperative cognitive dysfunction after cardiac surgery using cardiopulmonary bypass.
Methods: After IRB approval, 277 patients undergoing cardiac surgery were enrolled into this prospective, randomized, double-blinded placebo controlled clinical trial. Subjects were randomized to receive: (1) Lidocaine as a 1 mg/kg bolus followed by a continuous infusion through 48 hours postoperatively, or (2) Placebo bolus and infusion. Cognitive function was assessed preoperatively and again at 6 weeks and 1 year postoperatively. The effect of lidocaine on postoperative cognition was tested using multivariable regression modeling; P<0.05 was considered significant.
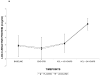
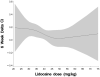
Results: Among the 241 allocated subjects (Lidocaine: n=114; Placebo: n=127), the incidence of cognitive deficit in the lidocaine group was 45.5% versus 45.7% in the placebo group (P=0.97). Multivariable analysis revealed a significant interaction between treatment group and diabetes, such that diabetic subjects receiving lidocaine were more likely to suffer cognitive decline (P=0.004). Secondary analysis identified total lidocaine dose (mg/kg) as a significant predictor of cognitive decline and also revealed a protective effect of lower dose lidocaine in nondiabetic subjects.
Conclusions: Lidocaine administered during and after cardiac surgery does not reduce the high rate of postoperative cognitive dysfunction. Higher doses of lidocaine and diabetic status were independent predictors of cognitive decline. Protective effects of lower dose lidocaine in nondiabetic subjects need to be further evaluated.
Figures









References
-
- Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. - PubMed
-
- Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68:369–375. - PubMed
-
- Zimpfer D, Czerny M, Vogt F, Schuch P, Kramer L, Wolner E, Grimm M. Neurocognitive deficit following coronary artery bypass grafting: A prospective study of surgical patients and nonsurgical controls. Ann Thorac Surg. 2004;78:513–518. discussion 518-519. - PubMed
-
- Stygall J, Newman SP, Fitzgerald G, Steed L, Mulligan K, Arrowsmith JE, Pugsley W, Humphries S, Harrison MJ. Cognitive change 5 years after coronary artery bypass surgery. Health Psychol. 2003;22:579–586. - PubMed
-
- Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63:581–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

