RUNX proteins in transcription factor networks that regulate T-cell lineage choice
- PMID: 19165227
- PMCID: PMC4231139
- DOI: 10.1038/nri2489
RUNX proteins in transcription factor networks that regulate T-cell lineage choice
Abstract
Recent research has uncovered complex transcription factor networks that control the processes of T-cell development and differentiation. RUNX (runt-related transcription factor) proteins are among the many factors that have crucial roles in these networks. In this Review, we examine the mechanisms by which RUNX complexes act together with other transcription factors, such as Th-POK (T-helper-inducing POZ/Kruppel-like factor) and GATA-binding protein 3 (GATA3) in determining the CD4/CD8 lineage choice of developing thymocytes. In addition, we discuss evidence indicating that RUNX complexes are also involved in the differentiation of effector T-cell subsets and that the molecular mechanisms by which RUNX proteins regulate T-cell fate decisions are conserved between the thymus and periphery.
Figures
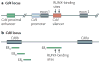



Comment in
-
Decision making during the conception and career of CD4+ T cells.Nat Rev Immunol. 2009 Feb;9(2):81-2. doi: 10.1038/nri2490. Nat Rev Immunol. 2009. PMID: 19172726
References
-
- Robey EA, et al. Thymic selection in CD8 transgenic mice supports an instructive model for commitment to a CD4 or CD8 lineage. Cell. 1991;64:99–107. - PubMed
-
- Davis CB, Killeen N, Crooks ME, Raulet D, Littman DR. Evidence for a stochastic mechanism in the differentiation of mature subsets of T lymphocytes. Cell. 1993;73:237–247. - PubMed
-
- Yasutomo K, Doyle C, Miele L, Fuchs C, Germain RN. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature. 2000;404:506–510. - PubMed
-
- Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. - PubMed
-
- Brugnera E, et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

