Rab11A-controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis
- PMID: 19165333
- PMCID: PMC2622761
- DOI: 10.1371/journal.ppat.1000270
Rab11A-controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis
Abstract
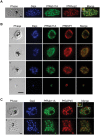
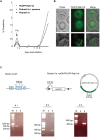
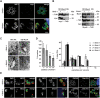
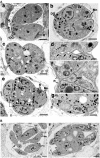
The final step during cell division is the separation of daughter cells, a process that requires the coordinated delivery and assembly of new membrane to the cleavage furrow. While most eukaryotic cells replicate by binary fission, replication of apicomplexan parasites involves the assembly of daughters (merozoites/tachyzoites) within the mother cell, using the so-called Inner Membrane Complex (IMC) as a scaffold. After de novo synthesis of the IMC and biogenesis or segregation of new organelles, daughters bud out of the mother cell to invade new host cells. Here, we demonstrate that the final step in parasite cell division involves delivery of new plasma membrane to the daughter cells, in a process requiring functional Rab11A. Importantly, Rab11A can be found in association with Myosin-Tail-Interacting-Protein (MTIP), also known as Myosin Light Chain 1 (MLC1), a member of a 4-protein motor complex called the glideosome that is known to be crucial for parasite invasion of host cells. Ablation of Rab11A function results in daughter parasites having an incompletely formed IMC that leads to a block at a late stage of cell division. A similar defect is observed upon inducible expression of a myosin A tail-only mutant. We propose a model where Rab11A-mediated vesicular traffic driven by an MTIP-Myosin motor is necessary for IMC maturation and to deliver new plasma membrane to daughter cells in order to complete cell division.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- D'Avino PP, Savoian MS, Capalbo L, Glover DM. RacGAP50C is sufficient to signal cleavage furrow formation during cytokinesis. J Cell Sci. 2006;119:4402–4408. - PubMed
-
- D'Avino PP, Savoian MS, Glover DM. Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J Cell Sci. 2005;118:1549–1558. - PubMed
-
- Jurgens G. Plant cytokinesis: fission by fusion. Trends Cell Biol. 2005;15:277–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

