Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct
- PMID: 19165513
- PMCID: PMC2672108
- DOI: 10.1007/s00775-009-0466-z
Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct
Abstract
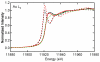
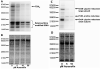
Clostridium difficile is a nosocomial pathogen whose incidence and importance are on the rise. Previous work in our laboratory characterized the central role of selenoenzyme-dependent Stickland reactions in C. difficile metabolism. In this work we have identified, using mass spectrometry, a stable complex formed upon reaction of auranofin (a gold-containing drug) with selenide in vitro. X-ray absorption spectroscopy supports the structure that we proposed on the basis of mass-spectrometric data. Auranofin potently inhibits the growth of C. difficile but does not similarly affect other clostridia that do not utilize selenoproteins to obtain energy. Moreover, auranofin inhibits the incorporation of radioisotope selenium ((75)Se) in selenoproteins in both Escherichia coli, the prokaryotic model for selenoprotein synthesis, and C. difficile without impacting total protein synthesis. Auranofin blocks the uptake of selenium and results in the accumulation of the auranofin-selenide adduct in the culture medium. Addition of selenium in the form of selenite or L-selenocysteine to the growth medium significantly reduces the inhibitory action of auranofin on the growth of C. difficile. On the basis of these results, we propose that formation of this complex and the subsequent deficiency in available selenium for selenoprotein synthesis is the mechanism by which auranofin inhibits C. difficile growth. This study demonstrates that targeting selenium metabolism provides a new avenue for antimicrobial development against C. difficile and other selenium-dependent pathogens.
Figures








References
-
- Kean WF, Hart L, Buchanan WW. British journal of rheumatology. 1997;36:560–572. - PubMed
-
- Messori L, Marcon G. Met Ions Biol Syst. 2004;41:279–304. - PubMed
-
- Becker K, Gromer S, Schirmer RH, Muller S. Eur J Biochem. 2000;267:6118–6125. - PubMed
-
- Gromer S, Arscott LD, Williams CH, Jr., Schirmer RH, Becker K. J Biol Chem. 1998;273:20096–20101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

