Substrate binding preferences and pka determinations of a nitrile hydratase model complex: variable solvent coordination to [(bmmp-TASN)Fe]OTf
- PMID: 19166306
- PMCID: PMC2754792
- DOI: 10.1021/ic802180d
Substrate binding preferences and pka determinations of a nitrile hydratase model complex: variable solvent coordination to [(bmmp-TASN)Fe]OTf
Abstract
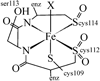
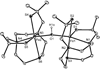
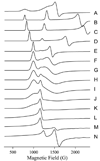
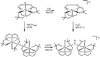
The five-coordinate iron-dithiolate complex (N,N'-4,7-bis-(2'-methyl-2'-mercatopropyl)-1-thia-4,7-diazacyclononane)iron(III), [LFe]+, has been isolated as the triflate salt from reaction of the previously reported LFeCl with thallium triflate. Spectroscopic characterization confirms an S = 1/2 ground state in non-coordinating solvents with room temperature microeff = 1.78 microB and electron paramagnetic resonance (EPR) derived g-values of g1 = 2.04, g2 = 2.02 and g3 = 2.01. [LFe]+ binds a variety of coordinating solvents resulting in six-coordinate complexes [LFe-solvent]+. In acetonitrile the low-spin [LFe-NCMe]+ (g1 = 2.27, g2 = 2.18, and g3 = 1.98) is in equilibrium with [LFe]+ with a binding constant of Keq = 4.7 at room temperature. Binding of H2O, DMF, methanol, DMSO, and pyridine to [LFe]+ yields high-spin six-coordinate complexes with EPR spectra that display significant strain in the rhombic zero-field splitting term E/D. Addition of 1 equiv of triflic acid to the previously reported diiron species (LFe)2O results in the formation of [(LFe)2OH]OTf, which has been characterized by X-ray crystallography. The aqueous chemistry of [LFe]+ reveals three distinct species as a function of pH: [LFe-OH2]+, [(LFe)2OH]OTf, and (LFe)2O. The pKa values for [LFe-OH2]+ and [(LFe)2OH]OTf are 5.4 +/- 0.1 and 6.52 +/- 0.05, respectively.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

