Ultrastructural changes of posterior lingual glands after hypoglossal denervation in hamsters
- PMID: 19166479
- PMCID: PMC2667924
- DOI: 10.1111/j.1469-7580.2008.01019.x
Ultrastructural changes of posterior lingual glands after hypoglossal denervation in hamsters
Abstract
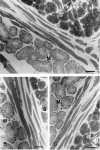
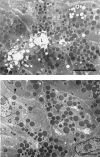
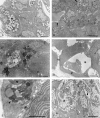
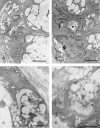
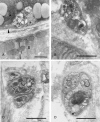
Posterior lingual glands consist of two sets of minor salivary glands that serve important functions in oral physiology. To investigate the hypothesis that the hypoglossal nerve provides sympathetic innervation to the posterior lingual glands, we examined ultrastructural changes in the glands following hypoglossal denervation. In the posterior deep lingual glands (of von Ebner), the serous acinar cells showed a decrease in the number of secretory granules and an increase in lipofuscin accumulation. The ratios of cells containing lipofuscin granules were 11.39, 36.49 and 50.46%, respectively, of the control, 3- and 7-day post-axotomy glands (P < 0.001). Intraepithelial phagocytotic activity was increased. The mucous acinar cells in the posterior superficial lingual glands (of Weber) also showed degenerative changes after hypoglossal denervation. One week after nerve transection, marked cytoplasmic vacuolation and fragmentation of organelles were frequently observed. Degenerative changes were also found in unmyelinated axons associated with the glands. We provide the first evidence of the structural and functional connections between the sympathetic component of the hypoglossal nerve and posterior lingual glands.
Figures
Similar articles
-
Evidence of neuroanatomical connection between the superior cervical ganglion and hypoglossal nerve in the hamster as revealed by tract-tracing and degeneration methods.J Anat. 2001 Apr;198(Pt 4):407-21. doi: 10.1046/j.1469-7580.2001.19840407.x. J Anat. 2001. PMID: 11327203 Free PMC article.
-
A high resolution sem study of human minor salivary glands.Eur J Morphol. 2000 Oct;38(4):219-26. doi: 10.1076/0924-3860(200010)38:4;1-o;ft219. Eur J Morphol. 2000. PMID: 10980671
-
Ultrastructural identification of a sympathetic component in the hypoglossal nerve of hamsters using experimental degeneration and horseradish peroxidase methods.Cells Tissues Organs. 2005;180(2):117-25. doi: 10.1159/000086752. Cells Tissues Organs. 2005. PMID: 16113540
-
Two types of inhibitory postsynaptic potentials in the hypoglossal motoneurons.Prog Neurobiol. 1993 Mar;40(3):385-411. doi: 10.1016/0301-0082(93)90016-l. Prog Neurobiol. 1993. PMID: 8441813 Review. No abstract available.
-
Cranial nerve XII: the hypoglossal nerve.Semin Neurol. 2009 Feb;29(1):45-52. doi: 10.1055/s-0028-1124022. Epub 2009 Feb 12. Semin Neurol. 2009. PMID: 19214932 Review.
Cited by
-
Occurrence of gustducin-immunoreactive cells in von Ebner's glands of guinea pigs.Histochem Cell Biol. 2013 Nov;140(5):567-74. doi: 10.1007/s00418-013-1094-9. Epub 2013 Apr 19. Histochem Cell Biol. 2013. PMID: 23604549 Free PMC article.
-
Biological aspects of the tongue morphology of wild-captive WWCPS rats: a histological, histochemical and ultrastructural study.Anat Sci Int. 2018 Sep;93(4):514-532. doi: 10.1007/s12565-018-0445-y. Epub 2018 Jun 14. Anat Sci Int. 2018. PMID: 29948977 Free PMC article.
-
Morphological evidences in circumvallate papilla and von Ebners' gland development in mice.Anat Cell Biol. 2011 Dec;44(4):274-83. doi: 10.5115/acb.2011.44.4.274. Epub 2011 Dec 30. Anat Cell Biol. 2011. PMID: 22254156 Free PMC article.
References
-
- Boshell JL. Effects of isoproterenol on the ultrastructure of pig parotid gland. Acta Anat (Basel) 1981;109:270–274. - PubMed
-
- Bowers CW, Zigmond RE. Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J Comp Neurol. 1979;185:381–392. - PubMed
-
- Bradley RM, Mistretta CM, Bates CA, Killackey HP. Transganglionic transport of HRP from the circumvallate papilla of the rat. Brain Res. 1985;361:154–161. - PubMed
-
- Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

