Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases
- PMID: 19166904
- PMCID: PMC3981543
- DOI: 10.1016/j.bbagen.2008.12.001
Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases
Abstract
Recent studies that alleles in the hemochromatosis gene may accelerate the onset of Alzheimer's disease by five years have validated interest in the model in which metals (particularly iron) accelerate disease course. Biochemical and biophysical measurements demonstrated the presence of elevated levels of neurotoxic copper zinc and iron in the brains of AD patients. Intracellular levels of APP holoprotein were shown to be modulated by iron by a mechanism that is similar to the translation control of the ferritin L- and H mRNAs by iron-responsive element (IRE) RNA stem loops in their 5' untranslated regions (5'UTRs). More recently a putative IRE-like sequence was hypothesized present in the Parkinsons's alpha synuclein (ASYN) transcript (see [A.L. Friedlich, R.E. Tanzi, J.T. Rogers, The 5'-untranslated region of Parkinson's disease alpha-synuclein messenger RNA contains a predicted iron responsive element, Mol. Psychiatry 12 (2007) 222-223. [6]]). Together with the demonstration of metal dependent translation of APP mRNA, the involvement of metals in the plaque of AD patients and of increased iron in striatal neurons in the substantia nigra (SN) of Parkinson's disease patients have stimulated the development of metal attenuating agents and iron chelators as a major new therapeutic strategy for the treatment of these neurodegenerative diseases. In the case of AD, metal based therapeutics may ultimately prove more cost effective than the use of an amyloid vaccine as the preferred anti-amyloid therapeutic strategy to ameliorate the cognitive decline of AD patients.
Figures

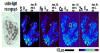
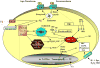



References
-
- Kosik K, Ahn J, Stein R, Yeh L. Discovery of compounds that will prevent tau pathology. J Mol Neurosci. 2002;19:261–266. - PubMed
-
- Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer's disease. J Biol Chem. 1996;271:18295–18298. - PubMed
-
- Hardy J. Framing β-amyloid. Nature Genetics. 1992;1:233–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

