Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography
- PMID: 19167082
- PMCID: PMC2695995
- DOI: 10.1016/j.ophtha.2008.10.006
Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography
Abstract
Purpose: Detect changes in the neurosensory retina using spectral-domain optical coherence tomography (SD OCT) imaging over drusen in age-related macular degeneration (AMD). Quantitative imaging biomarkers may aid in defining risk of disease progression.
Design: Cross-sectional, case-control study evaluating SD OCT testing in AMD.
Participants and controls: Seventeen eyes of 12 subjects with nonneovascular AMD and drusen and 17 eyes of 10 age-matched control subjects.
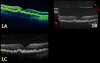
Methods: Spectral-domain OCT imaging across the fovea in the study eye with multiple 10- to 12-mm scans of 1000 A scans each.
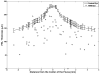
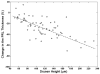
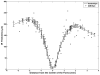
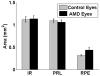
Main outcome measures: In summed SD OCT scans, the height of individual retinal layers either over drusen or at corresponding locations in the control eye and qualitative changes in retinal layers over drusen. Secondary measures included photoreceptor layer (PRL) area, inner retinal area, and retinal pigment epithelium (RPE)/drusen area.
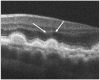
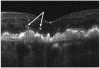
Results: The PRL was thinned over 97% of drusen, average PRL thickness was reduced by 27.5% over drusen compared with over a similar location in controls, and the finding of a difference was valid and significant (P=0.004). Photoreceptor outer segments were absent over at least 1 druse in 47% of eyes. Despite thinning of the PRL, inner retinal thickness remained unchanged. We observed 2 types of hyperreflective abnormalities in the neurosensory retina over drusen. Distinct hyperreflective speckled patterns occurred over drusen in 41% of AMD eyes and never in control eyes. A prominent hyperreflective haze was present in the photoreceptor nuclear layer over drusen in 67% of AMD eyes and more subtly in the photoreceptor nuclear layer in 18% of control eyes (no drusen).
Conclusions: With SD OCT as used in this study, we can easily detect and measure changes in PRL over drusen. Decreased PRL thickness over drusen suggests a degenerative process, with cell loss leading to decreased visual function. The hyperreflective foci overlying drusen are likely to represent progression of disease RPE cell migration into the retina and possible photoreceptor degeneration or glial scar formation. A longitudinal study using SD OCT to examine and measure the neurosensory retina over drusen will resolve the timeline of degenerative changes relative to druse formation.
Figures
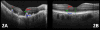
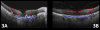


References
-
- Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. - PubMed
-
- Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia: the Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60. - PubMed
-
- Rohrer B, Guo Y, Kunchithapautham K, Gilkeson GS. Eliminating complement factor D reduces photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 2007;48:5282–9. - PubMed
-
- Wong RW, Richa DC, Hahn P, et al. Iron toxicity as a potential factor in AMD. Retina. 2007;27:997–1003. - PubMed
-
- Pauleikhoff D, Barondes MJ, Minassian D, et al. Drusen as risk factors in age-related macular disease. Am J Ophthalmol. 1990;109:38–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

