Assembly of ribosomes and spliceosomes: complex ribonucleoprotein machines
- PMID: 19167202
- PMCID: PMC2698946
- DOI: 10.1016/j.ceb.2009.01.003
Assembly of ribosomes and spliceosomes: complex ribonucleoprotein machines
Abstract
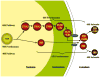
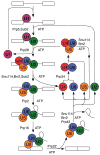
Ribosomes and spliceosomes are ribonucleoprotein nanomachines that catalyze translation of mRNA to synthesize proteins and splicing of introns from pre-mRNAs, respectively. Assembly of ribosomes involves more than 300 proteins and RNAs, and that of spliceosomes over 100 proteins and RNAs. Construction of these enormous ribonucleoprotein particles (RNPs) is a dynamic process, in which the nascent RNPs undergo numerous ordered rearrangements of RNA-RNA, RNA-protein, and protein-protein interactions. Here we outline similar principles that have emerged from studies of ribosome and spliceosome assembly. Constituents of both RNPs form subassembly complexes, which can simplify the task of assembly and segregate functions of assembly factors. Reorganization of RNP topology, and proofreading of proper assembly, are catalyzed by protein- or RNA-dependent ATPases or GTPases. Dynamics of intermolecular interactions may be facilitated or regulated by cycles of post-translational modifications. Despite this repertoire of tools, mistakes occur in RNP assembly or in processing of RNA substrates. Quality control mechanisms recognize and turnover misassembled RNPs and reject improper substrates.
Figures
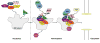
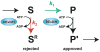
References
-
- Decatur W, Fournier MJ. RNA guided nucleotide modification of ribosomal and other RNAs. J Biol Chem. 2003;278:695–698. - PubMed
-
- Schafer T, Maco B, Petfalski E, Tollervey D, Bottcher B, Aebi M, Hurt E. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature. 2006;441:651–655. In vitro assays with purified preribosomes revealed that phosphorylation then dephosphorylation of ribosomal protein rpS3 caused it to be more stably associated with preribosomes. This cycle of modifications of rpS3, required for subunit biogenesis, was also correlated with structural rearrangements of the pre-40S particle, visible by cryo-EM. Mature 40S particles contain a “beak” structure, caused by protruding helix 33 of the 18S rRNA, whereas pre-40S particles lack the structure. The protein kinase Hrr25 was found to be associated with these preribosomes and required in vivo for this phosphorylation of rpS3 and maturation of pre-40S particles. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

