Inhibition of lactoperoxidase by its own catalytic product: crystal structure of the hypothiocyanate-inhibited bovine lactoperoxidase at 2.3-A resolution
- PMID: 19167310
- PMCID: PMC2716474
- DOI: 10.1016/j.bpj.2008.09.019
Inhibition of lactoperoxidase by its own catalytic product: crystal structure of the hypothiocyanate-inhibited bovine lactoperoxidase at 2.3-A resolution
Abstract
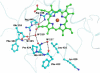
To the best of our knowledge, this is the first report on the structure of product-inhibited mammalian peroxidase. Lactoperoxidase is a heme containing an enzyme that catalyzes the inactivation of a wide range of microorganisms. In the presence of hydrogen peroxide, it preferentially converts thiocyanate ion into a toxic hypothiocyanate ion. Samples of bovine lactoperoxidase containing thiocyanate (SCN(-)) and hypothiocyanate (OSCN(-)) ions were purified and crystallized. The structure was determined at 2.3-A resolution and refined to R(cryst) and R(free) factors of 0.184 and 0.221, respectively. The determination of structure revealed the presence of an OSCN(-) ion at the distal heme cavity. The presence of OSCN(-) ions in crystal samples was also confirmed by chemical and spectroscopic analysis. The OSCN(-) ion interacts with the heme iron, Gln-105 N(epsilon1), His-109 N(epsilon2), and a water molecule W96. The sulfur atom of the OSCN(-) ion forms a hypervalent bond with a nitrogen atom of the pyrrole ring D of the heme moiety at an S-N distance of 2.8 A. The heme group is covalently bound to the protein through two ester linkages involving carboxylic groups of Glu-258 and Asp-108 and the modified methyl groups of pyrrole rings A and C, respectively. The heme moiety is significantly distorted from planarity, whereas pyrrole rings A, B, C, and D are essentially planar. The iron atom is displaced by approximately 0.2 A from the plane of the heme group toward the proximal site. The substrate channel resembles a long tunnel whose inner walls contain predominantly aromatic residues such as Phe-113, Phe-239, Phe-254, Phe-380, Phe-381, Phe-422, and Pro-424. A phosphorylated Ser-198 was evident at the surface, in the proximity of the calcium-binding channel.
Figures


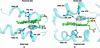




Similar articles
-
Structural evidence of substrate specificity in mammalian peroxidases: structure of the thiocyanate complex with lactoperoxidase and its interactions at 2.4 A resolution.J Biol Chem. 2009 May 29;284(22):14849-56. doi: 10.1074/jbc.M807644200. Epub 2009 Apr 1. J Biol Chem. 2009. PMID: 19339248 Free PMC article.
-
Crystal structure of lactoperoxidase at 2.4 A resolution.J Mol Biol. 2008 Feb 29;376(4):1060-75. doi: 10.1016/j.jmb.2007.12.012. Epub 2007 Dec 14. J Mol Biol. 2008. PMID: 18191143
-
Potassium-induced partial inhibition of lactoperoxidase: structure of the complex of lactoperoxidase with potassium ion at 2.20 Å resolution.J Biol Inorg Chem. 2021 Feb;26(1):149-159. doi: 10.1007/s00775-020-01844-6. Epub 2021 Jan 11. J Biol Inorg Chem. 2021. PMID: 33427997
-
Structural basis of activation of mammalian heme peroxidases.Prog Biophys Mol Biol. 2018 Mar;133:49-55. doi: 10.1016/j.pbiomolbio.2017.11.003. Epub 2017 Nov 22. Prog Biophys Mol Biol. 2018. PMID: 29174286 Review.
-
Lactoperoxidase: Properties, Functions, and Potential Applications.Int J Mol Sci. 2025 May 24;26(11):5055. doi: 10.3390/ijms26115055. Int J Mol Sci. 2025. PMID: 40507866 Free PMC article. Review.
Cited by
-
Enhanced Antibacterial Activity of Lactoperoxidase-Thiocyanate-Hydrogen Peroxide System in Reduced-Lactose Milk Whey.Int J Food Sci. 2019 Apr 23;2019:8013402. doi: 10.1155/2019/8013402. eCollection 2019. Int J Food Sci. 2019. PMID: 31179314 Free PMC article.
-
Quantitative N-glycoproteome analysis of bovine milk and yogurt.Curr Res Food Sci. 2022 Jan 12;5:182-190. doi: 10.1016/j.crfs.2022.01.003. eCollection 2022. Curr Res Food Sci. 2022. PMID: 35072106 Free PMC article.
-
Structural evidence of substrate specificity in mammalian peroxidases: structure of the thiocyanate complex with lactoperoxidase and its interactions at 2.4 A resolution.J Biol Chem. 2009 May 29;284(22):14849-56. doi: 10.1074/jbc.M807644200. Epub 2009 Apr 1. J Biol Chem. 2009. PMID: 19339248 Free PMC article.
-
T47D Cells Expressing Myeloperoxidase Are Able to Process, Traffic and Store the Mature Protein in Lysosomes: Studies in T47D Cells Reveal a Role for Cys319 in MPO Biosynthesis that Precedes Its Known Role in Inter-Molecular Disulfide Bond Formation.PLoS One. 2016 Feb 18;11(2):e0149391. doi: 10.1371/journal.pone.0149391. eCollection 2016. PLoS One. 2016. PMID: 26890638 Free PMC article.
-
Mode of binding of the antithyroid drug propylthiouracil to mammalian haem peroxidases.Acta Crystallogr F Struct Biol Commun. 2015 Mar;71(Pt 3):304-10. doi: 10.1107/S2053230X15001806. Epub 2015 Feb 19. Acta Crystallogr F Struct Biol Commun. 2015. Retraction in: Acta Crystallogr F Struct Biol Commun. 2015 Jun;71(Pt 6):804. doi: 10.1107/S2053230X15006962. PMID: 25760705 Free PMC article. Retracted.
References
-
- Harrison J.E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem. 1976;251:1371–1374. - PubMed
-
- Wever R., Kast W.M., Kasinoedin J.H., Boelens R. The peroxidation of thiocyanate catalyzed by myeloperoxidase and lactoperoxidase. Biochim. Biophys. Acta. 1982;709:212–219. - PubMed
-
- Bolscher B.G., Plat H., Wever R. Some properties of human eosinophil peroxidase, a comparison with other peroxidases. Biochim. Biophys. Acta. 1984;784:177–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

