Regulation of cell cytoskeleton and membrane mechanics by electric field: role of linker proteins
- PMID: 19167316
- PMCID: PMC2716454
- DOI: 10.1016/j.bpj.2008.09.035
Regulation of cell cytoskeleton and membrane mechanics by electric field: role of linker proteins
Abstract
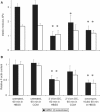
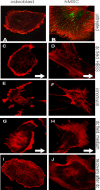
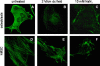
Cellular mechanics is known to play an important role in the cell homeostasis including proliferation, motility, and differentiation. Significant variation in the mechanical properties between different cell types suggests that control of the cell metabolism is feasible through manipulation of the cell mechanical parameters using external physical stimuli. We investigated the electrocoupling mechanisms of cellular biomechanics modulation by an electrical stimulation in two mechanically distinct cell types--human mesenchymal stem cells and osteoblasts. Application of a 2 V/cm direct current electric field resulted in approximately a twofold decrease in the cell elasticity and depleted intracellular ATP. Reduction in the ATP level led to inhibition of the linker proteins that are known to physically couple the cell membrane and cytoskeleton. The membrane separation from the cytoskeleton was confirmed by up to a twofold increase in the membrane tether length that was extracted from the cell membrane after an electrical stimulation. In comparison to human mesenchymal stem cells, the membrane-cytoskeleton attachment in osteoblasts was much stronger but, in response to the same electrical stimulation, the membrane detachment from the cytoskeleton was found to be more pronounced. The observed effects mediated by an electric field are cell type- and serum-dependent and can potentially be used for electrically assisted cell manipulation. An in-depth understanding and control of the mechanisms to regulate cell mechanics by external physical stimulus (e.g., electric field) may have great implications for stem cell-based tissue engineering and regenerative medicine.
Figures



Similar articles
-
Controlling cellular biomechanics of human mesenchymal stem cells.Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:2090-3. doi: 10.1109/IEMBS.2009.5333949. Annu Int Conf IEEE Eng Med Biol Soc. 2009. PMID: 19964578
-
Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells.Biophys J. 2007 Nov 15;93(10):3693-702. doi: 10.1529/biophysj.107.107797. Epub 2007 Aug 3. Biophys J. 2007. PMID: 17675345 Free PMC article.
-
Single-cell mechanics--An experimental-computational method for quantifying the membrane-cytoskeleton elasticity of cells.Acta Biomater. 2015 Nov;27:224-235. doi: 10.1016/j.actbio.2015.08.028. Epub 2015 Aug 20. Acta Biomater. 2015. PMID: 26300334
-
Cell mechanics: integrating cell responses to mechanical stimuli.Annu Rev Biomed Eng. 2007;9:1-34. doi: 10.1146/annurev.bioeng.9.060906.151927. Annu Rev Biomed Eng. 2007. PMID: 17461730 Review.
-
A new perspective for stem-cell mechanobiology: biomechanical control of stem-cell behavior and fate.Crit Rev Biomed Eng. 2010;38(5):393-433. doi: 10.1615/critrevbiomedeng.v38.i5.10. Crit Rev Biomed Eng. 2010. PMID: 21175407 Review.
Cited by
-
Quantitative Differentiation of Cell Surface-Bound and Internalized Cationic Gold Nanoparticles Using Mass Spectrometry.ACS Nano. 2016 Jul 26;10(7):6731-6. doi: 10.1021/acsnano.6b02105. Epub 2016 Jun 27. ACS Nano. 2016. PMID: 27337000 Free PMC article.
-
Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways.Tissue Eng Part B Rev. 2012 Dec;18(6):436-44. doi: 10.1089/ten.TEB.2012.0014. Epub 2012 Aug 6. Tissue Eng Part B Rev. 2012. PMID: 22741572 Free PMC article. Review.
-
Carbon Nanotube Uptake Changes the Biomechanical Properties of Human Lung Epithelial Cells in a Time-dependent Manner.J Mater Chem B. 2015;3:3983-3992. doi: 10.1039/C5TB00179J. J Mater Chem B. 2015. PMID: 26146559 Free PMC article.
-
Electroactive 4D Porous Scaffold Based on Conducting Polymer as a Responsive and Dynamic In Vitro Cell Culture Platform.ACS Appl Mater Interfaces. 2024 Feb 7;16(5):5613-5626. doi: 10.1021/acsami.3c16686. Epub 2024 Jan 26. ACS Appl Mater Interfaces. 2024. PMID: 38278772 Free PMC article.
-
Application of low-frequency alternating current electric fields via interdigitated electrodes: effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells.Tissue Eng Part C Methods. 2010 Dec;16(6):1377-86. doi: 10.1089/ten.TEC.2009.0751. Epub 2010 May 10. Tissue Eng Part C Methods. 2010. PMID: 20367249 Free PMC article.
References
-
- Boscolo P., Di Gioacchino M., Di Giampaolo L., Antonucci A., Di Luzio S. Combined effects of electromagnetic fields on immune and nervous responses. Int. J. Immunopathol. Pharmacol. 2007;20:59–63. - PubMed
-
- Mycielska M.E., Djamgoz M.B. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci. 2004;117:1631–1639. - PubMed
-
- Song B., Zhao M., Forrester J., McCaig C. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 2004;117:4681–4690. - PubMed
-
- Hotary K.B., Robinson K.R. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev. Biol. 1994;166:789–800. - PubMed
-
- Revest P.A., Jones H.C., Abbott N.J. Transendothelial electrical potential across pial vessels in anesthetized rats: a study of ion permeability and transport at the blood-brain barrier. Brain Res. 1994;652:76–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

