Role of the murine reprogramming factors in the induction of pluripotency
- PMID: 19167336
- PMCID: PMC3273494
- DOI: 10.1016/j.cell.2009.01.001
Role of the murine reprogramming factors in the induction of pluripotency
Abstract
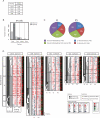
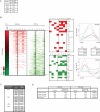
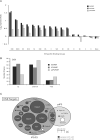
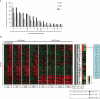
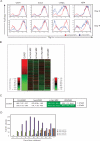
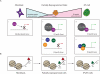
Induced pluripotent stem (iPS) cells can be obtained from fibroblasts upon expression of Oct4, Sox2, Klf4, and c-Myc. To understand how these factors induce pluripotency, we carried out genome-wide analyses of their promoter binding and expression in iPS and partially reprogrammed cells. We find that target genes of the four factors strongly overlap in iPS and embryonic stem (ES) cells. In partially reprogrammed cells, many genes co-occupied by c-Myc and any of the other three factors already show an ES cell-like binding and expression pattern. In contrast, genes that are specifically co-bound by Oct4, Sox2, and Klf4 in ES cells and encode pluripotency regulators severely lack binding and transcriptional activation. Among the four factors, c-Myc promotes the most ES cell-like transcription pattern when expressed individually in fibroblasts. These data uncover temporal and separable contributions of the four factors during the reprogramming process and indicate that ectopic c-Myc predominantly acts before pluripotency regulators are activated.
Figures

References
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
-
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. - PubMed
-
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

