Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis
- PMID: 19167487
- PMCID: PMC3576575
- DOI: 10.1016/j.cellsig.2009.01.015
Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis
Abstract
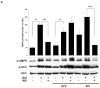
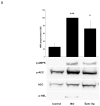
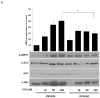
AMP-activated protein kinase (AMPK) is an important regulator of cellular energy status. In adipocytes, stimuli that increase intracellular cyclic AMP (cAMP) have also been shown to increase the activity of AMPK. The precise molecular mechanisms responsible for cAMP-induced AMPK activation are not clear. Phosphodiesterase 3B (PDE3B) is a critical regulator of cAMP signaling in adipocytes. Here we investigated the roles of PDE3B, PDE4, protein kinase B (PKB) and the exchange protein activated by cAMP 1 (Epac1), as well as lipolysis, in the regulation of AMPK in primary rat adipocytes. We demonstrate that the increase in phosphorylation of AMPK at T172 induced by the adrenergic agonist isoproterenol can be diminished by co-incubation with insulin. The diminishing effect of insulin on AMPK activation was reversed upon treatment with the PDE3B specific inhibitor OPC3911 but not with the PDE4 inhibitor Rolipram. Adenovirus-mediated overexpression of PDE3B and constitutively active PKB both resulted in greatly reduced isoproterenol-induced phosphorylation of AMPK at T172. Co-incubation of adipocytes with isoproterenol and the PKA inhibitor H89 resulted in a total ablation of lipolysis and a reduction in AMPK phosphorylation/activation. Stimulation of adipocytes with the Epac1 agonist 8-pCPT-2'O-Me-cAMP led to increased phosphorylation of AMPK at T172. The general lipase inhibitor Orlistat decreased isoproterenol-induced phosphorylation of AMPK at T172. This decrease corresponded to a reduction of lipolysis from adipocytes. Taken together, these data suggest that PDE3B and PDE4 regulate cAMP pools that affect the activation/phosphorylation state of AMPK and that the effects of cyclic AMP on AMPK involve Epac1, PKA and lipolysis.
Figures









Similar articles
-
Novel mechanisms of the regulation of protein kinase B in adipocytes; implications for protein kinase A, Epac, phosphodiesterases 3 and 4.Cell Signal. 2007 Jan;19(1):81-6. doi: 10.1016/j.cellsig.2006.05.024. Epub 2006 Jun 7. Cell Signal. 2007. PMID: 16839743
-
Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes.Cell Signal. 2006 Mar;18(3):382-90. doi: 10.1016/j.cellsig.2005.05.007. Epub 2005 Jun 14. Cell Signal. 2006. PMID: 15961276
-
Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP.Diabetes. 2002 Oct;51(10):2929-35. doi: 10.2337/diabetes.51.10.2929. Diabetes. 2002. PMID: 12351429
-
AKAP signaling complexes: getting to the heart of the matter.Trends Mol Med. 2006 Jul;12(7):317-23. doi: 10.1016/j.molmed.2006.05.008. Epub 2006 Jun 30. Trends Mol Med. 2006. PMID: 16809066 Review.
-
The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways.Eur J Cell Biol. 2006 Jul;85(7):593-602. doi: 10.1016/j.ejcb.2006.01.007. Epub 2006 Feb 7. Eur J Cell Biol. 2006. PMID: 16460834 Review.
Cited by
-
The glucose-lowering effects of the PDE4 inhibitors roflumilast and roflumilast-N-oxide in db/db mice.Diabetologia. 2012 Oct;55(10):2779-2788. doi: 10.1007/s00125-012-2632-z. Epub 2012 Jul 13. Diabetologia. 2012. PMID: 22790061
-
Sequence analysis and structure prediction of ABHD16A and the roles of the ABHD family members in human disease.Open Biol. 2018 May;8(5):180017. doi: 10.1098/rsob.180017. Open Biol. 2018. PMID: 29794032 Free PMC article. Review.
-
Xanthine-based KMUP-1 improves HDL via PPARγ/SR-B1, LDL via LDLRs, and HSL via PKA/PKG for hepatic fat loss.J Lipid Res. 2015 Nov;56(11):2070-84. doi: 10.1194/jlr.M057547. Epub 2015 Sep 8. J Lipid Res. 2015. PMID: 26351364 Free PMC article.
-
Dynamic changes in DICER levels in adipose tissue control metabolic adaptations to exercise.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23932-23941. doi: 10.1073/pnas.2011243117. Epub 2020 Sep 8. Proc Natl Acad Sci U S A. 2020. PMID: 32900951 Free PMC article.
-
Drug Candidates for Autoimmune Diseases.Pharmaceuticals (Basel). 2022 Apr 20;15(5):503. doi: 10.3390/ph15050503. Pharmaceuticals (Basel). 2022. PMID: 35631330 Free PMC article. Review.
References
-
- Carling D, Zammit VA, Hardie DG. FEBS Lett. 1987;223(2):217–222. - PubMed
-
- Davies SP, Hawley SA, Woods A, Carling D, Haystead TA, Hardie DG. Eur J Biochem. 1994;223(2):351–357. - PubMed
-
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Cell Metab. 2005;2(1):9–19. - PubMed
-
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Cell Metab. 2005;2(1):21–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

