Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas
- PMID: 19167838
- PMCID: PMC3659401
- DOI: 10.1016/j.ijrobp.2008.10.043
Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas
Abstract
Purpose: Preclinical studies suggest that inhibition of vascular endothelial growth factor (VEGF) improves glioma response to radiotherapy. Bevacizumab, a monoclonal antibody against VEGF, has shown promise in recurrent gliomas, but the safety and efficacy of concurrent bevacizumab with brain irradiation has not been extensively studied. The objectives of this study were to determine the safety and activity of this combination in malignant gliomas.
Methods and materials: After prior treatment with standard radiation therapy patients with recurrent glioblastoma (GBM) and anaplastic gliomas (AG) received bevacizumab (10 mg/kg intravenous) every 2 weeks of 28-day cycles until tumor progression. Patients also received 30 Gy of hypofractionated stereotactic radiotherapy (HFSRT) in five fractions after the first cycle of bevacizumab.
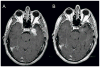
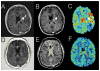
Results: Twenty-five patients (20 GBM, 5 AG; median age 56 years; median Karnofsky Performance Status 90) received a median of seven cycles of bevacizumab. One patient did not undergo HFSRT because overlap with prior radiotherapy would exceed the safe dose allowed to the optic chiasm. Three patients discontinued treatment because of Grade 3 central nervous system intratumoral hemorrhage, wound dehiscence, and bowel perforation. Other nonhematologic and hematologic toxicities were transient. No radiation necrosis was seen in these previously irradiated patients. For the GBM cohort, overall response rate was 50%, 6-month progression-free survival was 65%; median overall survival was 12.5 months, and 1-year survival was 54%.
Discussion: Bevacizumab with HFSRT is safe and well tolerated. Radiographic responses, duration of disease control, and survival suggest that this regimen is active in recurrent malignant glioma.
Trial registration: ClinicalTrials.gov NCT00595322.
Conflict of interest statement
Figures

References
-
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. - PubMed
-
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. - PubMed
-
- Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26:397–409. - PubMed
-
- Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. - PubMed
-
- Lamszus K, Ulbricht U, Matschke J, et al. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9:1399–1405. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

