Transgenic expression of aflatoxin aldehyde reductase (AKR7A1) modulates aflatoxin B1 metabolism but not hepatic carcinogenesis in the rat
- PMID: 19168568
- PMCID: PMC2675636
- DOI: 10.1093/toxsci/kfp003
Transgenic expression of aflatoxin aldehyde reductase (AKR7A1) modulates aflatoxin B1 metabolism but not hepatic carcinogenesis in the rat
Abstract
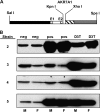
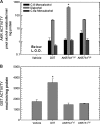
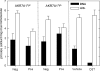
In both experimental animals and humans, aflatoxin B(1) (AFB(1)) is a potent hepatic toxin and carcinogen against which a variety of antioxidants and experimental or therapeutic drugs (e.g., oltipraz, related dithiolethiones, and various triterpenoids) protect from both acute toxicity and carcinogenesis. These agents induce several hepatic glutathione S-transferases (GST) as well as aldo-keto reductases (AKR) which are thought to contribute to protection. Studies were undertaken in transgenic rats to examine the role of one inducible enzyme, AKR7A1, for protection against acute and chronic actions of AFB(1) by enhancing detoxication of a reactive metabolite, AFB(1) dialdehyde, by reduction to alcohols. The AFB(1) dialdehyde forms adducts with protein amino groups by a Schiff base mechanism and these adducts have been theorized to be at least one cause of the acute toxicity of AFB(1) and to enhance carcinogenesis. A liver-specific AKR7A1 transgenic rat was constructed in the Sprague-Dawley strain and two lines, AKR7A1(Tg2) and AKR7A1(Tg5), were found to overexpress AKR7A1 by 18- and 8-fold, respectively. Rates of formation of AFB(1) alcohols, both in hepatic cytosols and as urinary excretion products, increased in the transgenic lines with AKR7A1(Tg2) being the highest. Neither line offered protection against acute AFB(1)-induced bile duct proliferation, a functional assessment of acute hepatotoxicity by AFB(1), nor did they protect against the formation of GST-P positive putative preneoplastic foci as a result of chronic exposure to AFB(1). These results imply that the prevention of protein adducts mediated by AKR are not critical to protection against AFB(1) tumorigenicity.
Figures


Comment in
-
Protection against aflatoxin B1 in rat--a new look at the link between toxicity, carcinogenicity, and metabolism.Toxicol Sci. 2009 May;109(1):1-3. doi: 10.1093/toxsci/kfp052. Epub 2009 Mar 16. Toxicol Sci. 2009. PMID: 19293422 No abstract available.
References
-
- Bodreddigari S, Jones LK, Egner PA, Groopman JD, Sutter CH, Roebuck BD, Guengerich FP, Kensler TW, Sutter TR. Protection against aflatoxin B1-induced cytotoxicity by expression of the cloned aflatoxin B1-aldehyde reductases rat AKR7A1 and human AKR7A3. Chem. Res. Toxicol. 2008;21:1134–1142. - PMC - PubMed
-
- Busby WF, Wogan GN. Aflatoxins. In: Searle CE, editor. Chemical Carcinogens. Washington, D.C.: American Chemical Society; 1984. pp. 945–1136.
-
- Crespi CL, Steimel DT, Aoyama T, Gelboin HV, Gonzalez FJ. Stable expression of human cytochrome P4501A2 cDNA in a human lymphoblastoid cell line: Role of the enzyme in the metabolic activation of aflatoxin B1. Mol. Carcinogenesis. 1990;3:5–8. - PubMed
-
- Eaton DL, Bammler TK. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol. Sci. 1999;49:156–164. - PubMed
-
- Eaton DL, Ramsdell HS, Neal GE. Biotransformation of aflatoxins. In: Eaton DL, Groopman JD, editors. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. New York: Academic Press; 1994. pp. 45–72.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

