A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors
- PMID: 19169250
- PMCID: PMC2694745
- DOI: 10.1038/nn.2249
A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors
Abstract
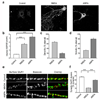
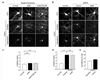
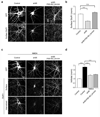
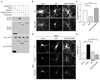
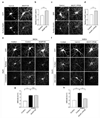
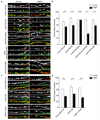
The endocytosis of AMPA receptors (AMPARs) underlies several forms of synaptic plasticity, including NMDA receptor (NMDAR)-dependent long-term depression (LTD), but the molecular mechanisms responsible for this trafficking remain unknown. We found that PSD-95, a major postsynaptic density protein, is important for NMDAR-triggered endocytosis of synaptic AMPARs in rat neuron cultures because of its binding to A kinase-anchoring protein 150 (AKAP150), a scaffold for specific protein kinases and phosphatases. Knockdown of PSD-95 with shRNA blocked NMDAR-triggered, but not constitutive or mGluR-triggered, endocytosis of AMPARs. Deletion of PSD-95's Src homology 3 and guanylate kinase-like domains, as well as a point mutation (L460P), both of which inhibit binding of PSD-95 to AKAP150, also blocked NMDAR-triggered AMPAR endocytosis. Furthermore, expression of a mutant AKAP150 that does not bind calcineurin inhibited this NMDAR-triggered trafficking event. Our results suggest that PSD-95's interaction with AKAP150 is critical for NMDAR-triggered AMPAR endocytosis and LTD, possibly because these scaffolds position calcineurin in the appropriate subsynaptic domain.
Figures


References
-
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. - PubMed
-
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. - PubMed
-
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:952–962. - PubMed
-
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Develop. Biol. 2007;23:613–643. - PubMed
-
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

