Different inflammatory responses are associated with Ureaplasma parvum-induced UTI and urolith formation
- PMID: 19171043
- PMCID: PMC2656517
- DOI: 10.1186/1471-2334-9-9
Different inflammatory responses are associated with Ureaplasma parvum-induced UTI and urolith formation
Abstract
Background: Epidemiologic studies show a strong association between Ureaplasmas and urogenital tract disease in humans. Since healthy humans can be colonized with Ureaplasmas, its role as a pathogen remains controversial. In order to begin to define the role of the host in disease, we developed a rodent model of urinary tract infection (UTI) using Fischer 344 (F344) rats. Animals were inoculated with sterile broth, 10(1), 10(3), 10(5), 10(7), or 10(9) log CFU of a rat-adapted strain of Ureaplasma parvum.
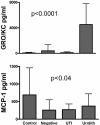
Results: Infected animals exhibited two distinct profiles, asymptomatic UTI and UTI complicated with struvite urolithiasis. Inoculum dose of U. parvum affected the incidence of UTI, and 50% to 57% of animals inoculated with >or= 10(7) CFU of U. parvum remained infected (p < 0.04). However, inoculum dose did not influence immune response to U. parvum. Asymptomatic UTI was characterized by a minimal immune response that was predominantly monocytic and lymphocytic, with limited lesions, and elevated urinary levels of IFN-gamma, IL-18 and MCP-1 (P <or= 0.02). UTI complicated with struvite formation was characterized by an exaggerated immune response that was mostly neutrophilic (P <or= 0.0001), with lesions that showed extensive uroepithelial hyperplasia (P <or= 0.0001), and a predominance of IL-1 alpha, IL-1 beta, and GRO/KC in the urine (P <or= 0.02). Animals with asymptomatic UTI also had a significantly high rate of kidney infection (P <or= 0.0005).
Conclusion: Complications associated with U. parvum infection are primarily dependent upon host-specific factors rather than Ureaplasma microbial load. The immune response in F344 rats is similar to that which occurs in humans with ureaplasmal associated disease. Therefore, this model of infection is a useful tool for elucidating U. parvum-host interactions that confer UTI and disease.
Figures






Similar articles
-
Complicated urinary tract infection is associated with uroepithelial expression of proinflammatory protein S100A8.Infect Immun. 2009 Oct;77(10):4265-74. doi: 10.1128/IAI.00458-09. Epub 2009 Aug 10. Infect Immun. 2009. PMID: 19667050 Free PMC article.
-
Ureaplasma parvum infection alters filamin A dynamics in host cells.BMC Infect Dis. 2011 Apr 20;11:101. doi: 10.1186/1471-2334-11-101. BMC Infect Dis. 2011. PMID: 21507248 Free PMC article.
-
Rat strains differ in susceptibility to Ureaplasma parvum-induced urinary tract infection and struvite stone formation.Infect Immun. 2006 Dec;74(12):6656-64. doi: 10.1128/IAI.00984-06. Epub 2006 Sep 18. Infect Immun. 2006. PMID: 16982825 Free PMC article.
-
Neonatal CNS infection and inflammation caused by Ureaplasma species: rare or relevant?Expert Rev Anti Infect Ther. 2015 Feb;13(2):233-48. doi: 10.1586/14787210.2015.999670. Expert Rev Anti Infect Ther. 2015. PMID: 25578885 Review.
-
Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update.Clin Perinatol. 2015 Dec;42(4):719-38. doi: 10.1016/j.clp.2015.08.003. Epub 2015 Oct 9. Clin Perinatol. 2015. PMID: 26593075 Free PMC article. Review.
Cited by
-
Extensive horizontal gene transfer in ureaplasmas from humans questions the utility of serotyping for diagnostic purposes.J Clin Microbiol. 2011 Aug;49(8):2818-26. doi: 10.1128/JCM.00637-11. Epub 2011 Jun 22. J Clin Microbiol. 2011. PMID: 21697330 Free PMC article.
-
The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy?PLoS One. 2012;7(1):e29856. doi: 10.1371/journal.pone.0029856. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22253806 Free PMC article.
-
Host genetic background impacts disease outcome during intrauterine infection with Ureaplasma parvum.PLoS One. 2012;7(8):e44047. doi: 10.1371/journal.pone.0044047. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952869 Free PMC article.
-
The Human Ureaplasma Species as Causative Agents of Chorioamnionitis.Clin Microbiol Rev. 2016 Dec 14;30(1):349-379. doi: 10.1128/CMR.00091-16. Print 2017 Jan. Clin Microbiol Rev. 2016. PMID: 27974410 Free PMC article. Review.
-
Is Non-Chlamydial Non-Gonococcal Urethritis Associated with Significant Clinical Complications in Men? A Systematic Review.Curr Urol. 2020 Mar;14(1):1-13. doi: 10.1159/000499266. Epub 2020 Mar 20. Curr Urol. 2020. PMID: 32398991 Free PMC article. Review.
References
-
- Skerk V, Marekovic I, Markovinovic L, Begovac J, Skerk V, Barsic N, Majdak-Gluhinic V. Comparative randomized pilot study of azithromycin and doxycycline efficacy and tolerability in the treatment of prostate infection caused by Ureaplasma urealyticum. Chemotherapy. 2006;52:9–11. doi: 10.1159/000090234. - DOI - PubMed
-
- Viscardi RM, Atamas SP, Luzina IG, Hasday JD, He JR, Sime PJ, Coalson JJ, Yoder BA. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatric research. 2006;60:141–146. doi: 10.1203/01.pdr.0000228322.73777.05. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

