Analysis of a conserved RGE/RGD motif in HCV E2 in mediating entry
- PMID: 19171049
- PMCID: PMC2637243
- DOI: 10.1186/1743-422X-6-12
Analysis of a conserved RGE/RGD motif in HCV E2 in mediating entry
Abstract
Background: Hepatitis C virus (HCV) encodes two transmembrane glycoproteins E1 and E2 which form a heterodimer. E1 is believed to mediate fusion while E2 has been shown to bind cellular receptors. It is clear that HCV uses a multi-receptor complex to gain entry into susceptible cells, however key elements of this complex remain elusive. In this study, the role of a highly conserved RGE/RGD motif of HCV E2 glycoprotein in viral entry was examined. The effect of each substitution mutation in this motif was tested by challenging susceptible cell lines with mutant HCV E1E2 pseudotyped viruses generated using a lentiviral system (HCVpp). In addition to assaying infectivity, producer cell expression and HCVpp incorporation of HCV E2 proteins, CD81 binding profiles, and conformation of mutants were examined.
Results: Based on these characteristics, mutants either displayed wt characteristics (high infectivity [> or = 90% of wt HCVpp], CD81 binding, E1E2 expression, and incorporation into viral particles and proper conformation) or very low infectivity (< or = 20% of wt HCVpp). Only amino acid substitutions of the 3rd position (D or E) resulted in wt characteristics as long as the negative charge was maintained or a neutral alanine was introduced. A change in charge to a positive lysine, disrupted HCVpp infectivity at this position.
Conclusion: Although most amino acid substitutions within this conserved motif displayed greatly reduced HCVpp infectivity, they retained soluble CD81 binding, proper E2 conformation, and incorporation into HCVpp. Our results suggest that although RGE/D is a well-defined integrin binding motif, in this case the role of these three hyperconserved amino acids does not appear to be integrin binding. As the extent of conservation of this region extends well beyond these three amino acids, we speculate that this region may play an important role in the structure of HCV E2 or in mediating the interaction with other factor(s) during viral entry.
Figures

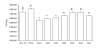


Similar articles
-
Dissecting the role of putative CD81 binding regions of E2 in mediating HCV entry: putative CD81 binding region 1 is not involved in CD81 binding.Virol J. 2008 Mar 20;5:46. doi: 10.1186/1743-422X-5-46. Virol J. 2008. PMID: 18355410 Free PMC article.
-
Mutational analysis of the hepatitis C virus E1 glycoprotein in retroviral pseudoparticles and cell-culture-derived H77/JFH1 chimeric infectious virus particles.J Viral Hepat. 2009 Sep;16(9):621-32. doi: 10.1111/j.1365-2893.2009.01111.x. Epub 2009 Mar 11. J Viral Hepat. 2009. PMID: 19302336 Free PMC article.
-
A conserved Gly436-Trp-Leu-Ala-Gly-Leu-Phe-Tyr motif in hepatitis C virus glycoprotein E2 is a determinant of CD81 binding and viral entry.J Virol. 2006 Aug;80(16):7844-53. doi: 10.1128/JVI.00029-06. J Virol. 2006. PMID: 16873241 Free PMC article.
-
Functional hepatitis C virus envelope glycoproteins.Biol Cell. 2004 Aug;96(6):413-20. doi: 10.1016/j.biolcel.2004.03.008. Biol Cell. 2004. PMID: 15325070 Review.
-
Hepatitis C virus entry: potential receptors and their biological functions.J Gen Virol. 2006 May;87(Pt 5):1075-1084. doi: 10.1099/vir.0.81646-0. J Gen Virol. 2006. PMID: 16603507 Review.
Cited by
-
An alternative view of the proposed alternative activities of hemopexin.Protein Sci. 2011 May;20(5):791-805. doi: 10.1002/pro.616. Epub 2011 Mar 30. Protein Sci. 2011. PMID: 21404362 Free PMC article. Review.
-
Integrin αvβ1 Modulation Affects Subtype B Avian Metapneumovirus Fusion Protein-mediated Cell-Cell Fusion and Virus Infection.J Biol Chem. 2016 Jul 8;291(28):14815-25. doi: 10.1074/jbc.M115.711382. Epub 2016 May 16. J Biol Chem. 2016. PMID: 27226547 Free PMC article.
-
Roles of the putative integrin-binding motif of the human metapneumovirus fusion (f) protein in cell-cell fusion, viral infectivity, and pathogenesis.J Virol. 2014 Apr;88(8):4338-52. doi: 10.1128/JVI.03491-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478423 Free PMC article.
-
Structural and Functional Determinants of Rodent and Human Surfactant Protein A: A Synthesis of Binding and Computational Data.Front Immunol. 2019 Nov 7;10:2613. doi: 10.3389/fimmu.2019.02613. eCollection 2019. Front Immunol. 2019. PMID: 31781112 Free PMC article.
-
Identification of interactions in the E1E2 heterodimer of hepatitis C virus important for cell entry.J Biol Chem. 2011 Jul 8;286(27):23865-76. doi: 10.1074/jbc.M110.213942. Epub 2011 May 9. J Biol Chem. 2011. PMID: 21555519 Free PMC article.
References
-
- Lindenbach BD, Rice CM. Flaviviridae: The viruses and their replication. In: Knipe DM, Howley PM, editor. Field's Virology. 4. Vol. 2. Philadelphia: Pippincott Williams & Wilkins; 2001. pp. 991–1041.
-
- Cocquerel L, Voisset C, Dubuisson J. Hepatitis C virus entry: potential receptors and their biological functions. J Gen Virol. 2006;87:1075–84. - PubMed
-
- Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, Scarselli E, Cortese R, Nicosia A, Cosset FL. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624–30. - PubMed
-
- Diedrich G. How does hepatitis C virus enter cells? Febs J. 2006;273:3871–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

