Mechanical compression attenuates normal human bronchial epithelial wound healing
- PMID: 19171062
- PMCID: PMC2672070
- DOI: 10.1186/1465-9921-10-5
Mechanical compression attenuates normal human bronchial epithelial wound healing
Abstract
Background: Airway narrowing associated with chronic asthma results in the transmission of injurious compressive forces to the bronchial epithelium and promotes the release of pro-inflammatory mediators and the denudation of the bronchial epithelium. While the individual effects of compression or denudation are well characterized, there is no data to elucidate how these cells respond to the application of mechanical compression in the presence of a compromised epithelial layer.
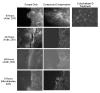
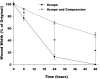
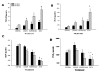
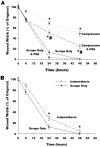
Methods: Accordingly, differentiated normal human bronchial epithelial cells were exposed to one of four conditions: 1) unperturbed control cells, 2) single scrape wound only, 3) static compression (6 hours of 30 cmH2O), and 4) 6 hours of static compression after a scrape wound. Following treatment, wound closure rate was recorded, media was assayed for mediator content and the cytoskeletal network was fluorescently labeled.
Results: We found that mechanical compression and scrape injury increase TGF-beta2 and endothelin-1 secretion, while EGF content in the media is attenuated with both injury modes. The application of compression after a pre-existing scrape wound augmented these observations, and also decreased PGE2 media content. Compression stimulated depolymerization of the actin cytoskeleton and significantly attenuated wound healing. Closure rate was partially restored with the addition of exogenous PGE2, but not EGF.
Conclusion: Our results suggest that mechanical compression reduces the capacity of the bronchial epithelium to close wounds, and is, in part, mediated by PGE2 and a compromised cytoskeleton.
Figures


Similar articles
-
Epithelial-derived TGF-beta2 modulates basal and wound-healing subepithelial matrix homeostasis.Am J Physiol Lung Cell Mol Physiol. 2006 Dec;291(6):L1277-85. doi: 10.1152/ajplung.00057.2006. Epub 2006 Aug 4. Am J Physiol Lung Cell Mol Physiol. 2006. PMID: 16891397
-
[Effects of airway epithelium injury on the transdifferentiation of sub-epithelial fibroblasts and its role in the development of airway hyperresponsiveness in asthma].Zhonghua Jie He He Hu Xi Za Zhi. 2005 Oct;28(10):698-703. Zhonghua Jie He He Hu Xi Za Zhi. 2005. PMID: 16255956 Chinese.
-
Wound-induced TGF-β1 and TGF-β2 enhance airway epithelial repair via HB-EGF and TGF-α.Biochem Biophys Res Commun. 2011 Aug 19;412(1):109-14. doi: 10.1016/j.bbrc.2011.07.054. Epub 2011 Jul 23. Biochem Biophys Res Commun. 2011. PMID: 21802406
-
The role of epithelial injury and repair in the origins of asthma.Curr Opin Allergy Clin Immunol. 2007 Feb;7(1):63-8. doi: 10.1097/ACI.0b013e328013d61b. Curr Opin Allergy Clin Immunol. 2007. PMID: 17218813 Review.
-
[The bronchial epithelium--more than a mechanical protection. The role of bronchial epithelial cells in defective healing has been determined].Lakartidningen. 1999 Oct 13;96(41):4419-20, 4423-5. Lakartidningen. 1999. PMID: 10553321 Review. Swedish.
Cited by
-
Differential β3 Integrin Expression Regulates the Response of Human Lung and Cardiac Fibroblasts to Extracellular Matrix and Its Components.Tissue Eng Part A. 2015 Aug;21(15-16):2195-205. doi: 10.1089/ten.TEA.2014.0337. Epub 2015 Jun 3. Tissue Eng Part A. 2015. PMID: 25926101 Free PMC article.
-
A microphysiological model of the bronchial airways reveals the interplay of mechanical and biochemical signals in bronchospasm.Nat Biomed Eng. 2019 Jul;3(7):532-544. doi: 10.1038/s41551-019-0366-7. Epub 2019 Mar 11. Nat Biomed Eng. 2019. PMID: 31150010 Free PMC article.
-
EGF-induced bronchial epithelial cells drive neutrophil chemotactic and anti-apoptotic activity in asthma.PLoS One. 2013 Sep 11;8(9):e72502. doi: 10.1371/journal.pone.0072502. eCollection 2013. PLoS One. 2013. PMID: 24039773 Free PMC article.
-
Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome?Am J Respir Cell Mol Biol. 2011 Apr;44(4):465-73. doi: 10.1165/rcmb.2009-0453OC. Epub 2010 Jun 4. Am J Respir Cell Mol Biol. 2011. PMID: 20525805 Free PMC article.
References
-
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. - PubMed
-
- Howarth PH, Wilson J, Djukanovic R, Wilson S, Britten K, Walls A, Roche WR, Holgate ST. Airway inflammation and atopic asthma: a comparative bronchoscopic investigation. Int Arch Allergy Appl Immunol. 1991;94(1–4):266–269. - PubMed
-
- Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998;102(5):783–788. - PubMed
-
- Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1(8637):520–524. - PubMed

