A circuit supporting concentration-invariant odor perception in Drosophila
- PMID: 19171076
- PMCID: PMC2656214
- DOI: 10.1186/jbiol108
A circuit supporting concentration-invariant odor perception in Drosophila
Abstract
Background: Most odors are perceived to have the same quality over a large concentration range, but the neural mechanisms that permit concentration-invariant olfactory perception are unknown. In larvae of the vinegar fly Drosophila melanogaster, odors are sensed by an array of 25 odorant receptors expressed in 21 olfactory sensory neurons (OSNs). We investigated how subsets of larval OSNs with overlapping but distinct response properties cooperate to mediate perception of a given odorant across a range of concentrations.
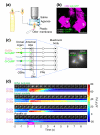
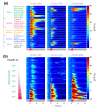
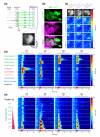
Results: Using calcium imaging, we found that ethyl butyrate, an ester perceived by humans as fruity, activated three OSNs with response thresholds that varied across three orders of magnitude. Whereas wild-type larvae were strongly attracted by this odor across a 500-fold range of concentration, individuals with only a single functional OSN showed attraction across a narrower concentration range corresponding to the sensitivity of each ethyl butyrate-tuned OSN. To clarify how the information carried by different OSNs is integrated by the olfactory system, we characterized the response properties of local inhibitory interneurons and projection neurons in the antennal lobe. Local interneurons only responded to high ethyl butyrate concentrations upon summed activation of at least two OSNs. Projection neurons showed a reduced response to odors when summed input from two OSNs impinged on the circuit compared to when there was only a single functional OSN.
Conclusions: Our results show that increasing odor concentrations induce progressive activation of concentration-tuned olfactory sensory neurons and concomitant recruitment of inhibitory local interneurons. We propose that the interplay of combinatorial OSN input and local interneuron activation allows animals to remain sensitive to odors across a large range of stimulus intensities.
Figures




References
-
- Wilson DA, Stevenson RJ. Learning to Smell: Olfactory Perception from Neurobiology to Behavior. Baltimore: Johns Hopkins University Press; 2006. pp. 64–75.
-
- Wachowiak M, Cohen LB, Zochowski MR. Distributed and concentration-invariant spatial representations of odorants by receptor neuron input to the turtle olfactory bulb. J Neurophysiol. 2002;87:1035–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

