Truncated dystrophins can influence neuromuscular synapse structure
- PMID: 19171194
- PMCID: PMC2826111
- DOI: 10.1016/j.mcn.2008.12.011
Truncated dystrophins can influence neuromuscular synapse structure
Abstract
Duchenne muscular dystrophy (DMD) is characterized by muscle degeneration and structural defects in the neuromuscular synapse that are caused by mutations in dystrophin. Whether aberrant neuromuscular synapse structure is an indirect consequence of muscle degeneration or a direct result of loss of dystrophin function is not known. Rational design of truncated dystrophins has enabled the design of expression cassettes highly effective at preventing muscle degeneration in mouse models of DMD using gene therapy. Here we examined the functional capacity of a minidystrophin (minidysGFP) and a microdystrophin (microdystrophin(DeltaR4-R23)) transgene on the maturation and maintenance of neuromuscular junctions (NMJ) in mdx mice. We found that minidysGFP prevents fragmentation and the loss of postsynaptic folds at the NMJ. In contrast, microdystrophin (DeltaR4-R23) was unable to prevent synapse fragmentation in the limb muscles despite preventing muscle degeneration, although fragmentation was observed to temporally correlate with the formation of ringed fibers. Surprisingly, microdystrophin(DeltaR4-R23) increased the length of synaptic folds in the diaphragm muscles of mdx mice independent of muscle degeneration or the formation of ringed fibers. We also demonstrate that the number and depth of synaptic folds influences the density of voltage-gated sodium channels at the neuromuscular synapse in mdx, microdystrophin(DeltaR4-R23)/mdx and mdx:utrophin double knockout mice. Together, these data suggest that maintenance of the neuromuscular synapse is governed through its lateral association with the muscle cytoskeleton, and that dystrophin has a direct role in promoting the maturation of synaptic folds to allow more sodium channels into the junction.
Figures
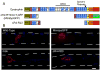



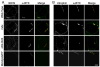
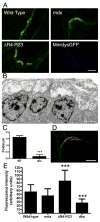
References
-
- Abmayr S, Chamberlain JS. The structure and function of dystrophin. In: Winder S, editor. The Molecular Mechanisms of Muscular Dystrophy. Landes Bioscience; Georgetown: 2006.
-
- Anderson JL, Head SI, Rae C, Morley JW. Brain function in Duchenne muscular dystrophy. Brain. 2002;125:4–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

