Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome
- PMID: 19171637
- PMCID: PMC2752886
- DOI: 10.1542/peds.2007-2781
Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome
Abstract
Objective: The purpose of this work was to evaluate therapy for patent ductus arteriosus as a risk factor for death or neurodevelopmental impairment at 18 to 22 months, bronchopulmonary dysplasia, or necrotizing enterocolitis in extremely low birth weight infants.
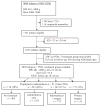
Methods: We studied infants in the National Institute of Child Health and Human Development Neonatal Research Network Generic Data Base born between 2000 and 2004 at 23 to 28 weeks' gestation and at <1000-g birth weight with patent ductus arteriosus. Patent ductus arteriosus therapy was evaluated as a risk factor for outcomes in bivariable and multivariable analyses.
Results: Treatment for subjects with patent ductus arteriosus (n = 2838) included 403 receiving supportive treatment only, 1525 treated with indomethacin only, 775 with indomethacin followed by secondary surgical closure, and 135 treated with primary surgery. Patients who received supportive therapy for patent ductus arteriosus did not differ from subjects treated with indomethacin only for any of the outcomes of interest. Compared with indomethacin treatment only, patients undergoing primary or secondary surgery were smaller and more premature. When compared with indomethacin alone, primary surgery was associated with increased adjusted odds for neurodevelopmental impairment and bronchopulmonary dysplasia in multivariable logistic regression. Secondary surgical closure was associated with increased odds for neurodevelopmental impairment and increased adjusted odds for bronchopulmonary dysplasia but decreased adjusted odds for death. Risk of necrotizing enterocolitis did not differ among treatments. Indomethacin prophylaxis did not significantly modify these results.
Conclusions: Our results suggest that infants treated with primary or secondary surgery for patent ductus arteriosus may be at increased risk for poor short- and long-term outcomes compared with those treated with indomethacin. Prophylaxis with indomethacin in the first 24 hours of life did not modify the subsequent outcomes of patent ductus arteriosus therapy.
Figures
Similar articles
-
Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study.J Pediatr Gastroenterol Nutr. 2005 Feb;40(2):184-8. doi: 10.1097/00005176-200502000-00019. J Pediatr Gastroenterol Nutr. 2005. PMID: 15699694
-
Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants.Pediatrics. 2008 Jan;121(1):142-7. doi: 10.1542/peds.2007-0925. Pediatrics. 2008. PMID: 18166568
-
Association between early echocardiography, therapy for patent ductus arteriosus, and outcomes in very low birth weight infants.Cardiol Young. 2017 Nov;27(9):1732-1739. doi: 10.1017/S1047951117001081. Epub 2017 Jun 19. Cardiol Young. 2017. PMID: 28625190
-
Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants.Cochrane Database Syst Rev. 2020 Jan 27;1(1):CD004213. doi: 10.1002/14651858.CD004213.pub5. Cochrane Database Syst Rev. 2020. PMID: 31985838 Free PMC article.
-
[Ibuprofen--a new application for pharmacological closure of patent ductus arteriosus in preterm infants. Preliminary report].Med Wieku Rozwoj. 2000;4(2 Suppl 3):65-71. Med Wieku Rozwoj. 2000. PMID: 11328971 Review. Polish.
Cited by
-
Surgical ligation of patent ductus arteriosus in preterm neonates weighing less than 1500g: a 9-year single center experience.J Cardiothorac Surg. 2020 Jun 17;15(1):144. doi: 10.1186/s13019-020-01191-2. J Cardiothorac Surg. 2020. PMID: 32552772 Free PMC article.
-
Comparison of Various Pharmacologic Agents in the Management of Hemodynamically Significant Patent Ductus Arteriosus in Preterm: A Network Meta-Analysis and Risk-Benefit Analysis.Biomed Hub. 2022 Oct 24;7(3):125-145. doi: 10.1159/000526318. eCollection 2022 Sep-Dec. Biomed Hub. 2022. PMID: 36465804 Free PMC article. Review.
-
Association between endotypes of prematurity and pharmacological closure of patent ductus arteriosus: A systematic review and meta-analysis.Front Pediatr. 2023 Mar 3;11:1078506. doi: 10.3389/fped.2023.1078506. eCollection 2023. Front Pediatr. 2023. PMID: 36937978 Free PMC article.
-
Targeted neonatal echocardiography for patent ductus arteriosus in neonates reduces the surgical ligation rate without affecting healthcare outcomes.Transl Pediatr. 2023 Feb 28;12(2):137-145. doi: 10.21037/tp-22-147. Epub 2023 Feb 1. Transl Pediatr. 2023. PMID: 36891358 Free PMC article.
-
Association of Antegrade Pulmonary Artery Diastolic Velocity with Spontaneous Closure of the Patent Ductus Arteriosus in Extremely Low-Birth-Weight Infants.Am J Perinatol. 2015 Nov;32(13):1217-24. doi: 10.1055/s-0035-1554795. Epub 2015 Jun 9. Am J Perinatol. 2015. PMID: 26058372 Free PMC article.
References
-
- Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol. 2007;196(2):147.e1–147.e8. - PubMed
-
- Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network 1996–1997. Pediatrics. 2000;106(5):1070–1079. - PubMed
-
- Itabashi K, Ohno T, Nishida H. Indomethacin responsiveness of patent ductus arteriosus and renal abnormalities in preterm infants treated with indomethacin. J Pediatr. 2003;143(2):203–207. - PubMed
-
- Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with PDA: results of a national collaborative study. J Pediatr. 1983;102(6):895–906. - PubMed
Publication types
MeSH terms
Grants and funding
- U10 HD27851/HD/NICHD NIH HHS/United States
- U10 HD40492/HD/NICHD NIH HHS/United States
- U10 HD021364/HD/NICHD NIH HHS/United States
- U10 HD21397,/HD/NICHD NIH HHS/United States
- U10 HD40521/HD/NICHD NIH HHS/United States
- M01 RR633/RR/NCRR NIH HHS/United States
- U10 HD21385/HD/NICHD NIH HHS/United States
- U10 HD040498/HD/NICHD NIH HHS/United States
- M01 RR006022/RR/NCRR NIH HHS/United States
- U10 HD027856/HD/NICHD NIH HHS/United States
- U10 HD40689/HD/NICHD NIH HHS/United States
- U10 HD021373/HD/NICHD NIH HHS/United States
- U10 HD021385/HD/NICHD NIH HHS/United States
- M01 RR44/RR/NCRR NIH HHS/United States
- U10 HD053119/HD/NICHD NIH HHS/United States
- U01 HD036790/HD/NICHD NIH HHS/United States
- U10 HD21364/HD/NICHD NIH HHS/United States
- U10 HD34216/HD/NICHD NIH HHS/United States
- U10 HD027880/HD/NICHD NIH HHS/United States
- M01 RR70/RR/NCRR NIH HHS/United States
- U10 HD040521/HD/NICHD NIH HHS/United States
- U10 HD27880/HD/NICHD NIH HHS/United States
- M01 RR008084/RR/NCRR NIH HHS/United States
- M01 RR125/RR/NCRR NIH HHS/United States
- U10 HD27904/HD/NICHD NIH HHS/United States
- U10 HD040461/HD/NICHD NIH HHS/United States
- U10 HD40498/HD/NICHD NIH HHS/United States
- U10 HD27871/HD/NICHD NIH HHS/United States
- M01 RR016587/RR/NCRR NIH HHS/United States
- M01 RR7122/RR/NCRR NIH HHS/United States
- U10 HD040689/HD/NICHD NIH HHS/United States
- U10 HD040492/HD/NICHD NIH HHS/United States
- U10 HD027853/HD/NICHD NIH HHS/United States
- U10 HD027904/HD/NICHD NIH HHS/United States
- U10 HD021397/HD/NICHD NIH HHS/United States
- U10 HD27856/HD/NICHD NIH HHS/United States
- U10 HD40461/HD/NICHD NIH HHS/United States
- U10 HD27853/HD/NICHD NIH HHS/United States
- M01 RR39/RR/NCRR NIH HHS/United States
- M01 RR30/RR/NCRR NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U10 HD027871/HD/NICHD NIH HHS/United States
- M01 RR007122/RR/NCRR NIH HHS/United States
- U10 HD027851/HD/NICHD NIH HHS/United States
- M01 RR80/RR/NCRR NIH HHS/United States
- M01 RR16587/RR/NCRR NIH HHS/United States
- U01 HD36790/HD/NICHD NIH HHS/United States
- M01 RR 6022/RR/NCRR NIH HHS/United States
- M01 RR750/RR/NCRR NIH HHS/United States
- M01 RR8084/RR/NCRR NIH HHS/United States
- U10 HD21373/HD/NICHD NIH HHS/United States
- M01 RR32/RR/NCRR NIH HHS/United States
- U10 HD034216/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical