A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist
- PMID: 19171674
- PMCID: PMC2684935
- DOI: 10.1124/mol.108.053439
A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist
Abstract
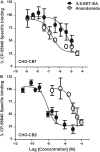
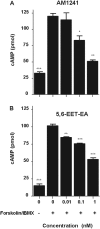
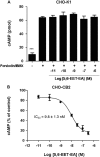
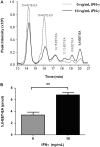
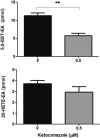
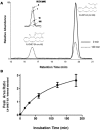
Oxidation of the endocannabinoid anandamide by cytochrome P450 (P450) enzymes has the potential to affect signaling pathways within the endocannabinoid system and pharmacological responses to novel drug candidates targeting this system. We previously reported that the human cytochromes P450 2D6, 3A4, and 4F2 are high-affinity, high-turnover anandamide oxygenases in vitro, forming the novel metabolites hydroxyeicosatetraenoic acid ethanolamides and epoxyeicosatrienoic acid ethanolamides. The objective of this study was to investigate the possible biological significance of these metabolic pathways. We report that the 5,6-epoxide of anandamide, 5,6-epoxyeicosatrienoic acid ethanolamide (5,6-EET-EA), is a potent and selective cannabinoid receptor 2 (CB2) agonist. The K(i) values for the binding of 5,6-EET-EA to membranes from Chinese hamster ovary (CHO) cells expressing either recombinant human CB1 or CB2 receptor were 11.4 microM and 8.9 nM, respectively. In addition, 5,6-EET-EA inhibited the forskolin-stimulated accumulation of cAMP in CHO cells stably expressing the CB2 receptor (IC(50) = 9.8 +/- 1.3 nM). Within the central nervous system, the CB2 receptor is expressed on activated microglia and is a potential therapeutic target for neuroinflammation. BV-2 microglial cells stimulated with low doses of interferon-gamma exhibited an increased capacity for converting anandamide to 5,6-EET-EA, which correlated with increased protein expression of microglial P450 4F and 3A isoforms. Finally, we demonstrate that 5,6-EET-EA is more stable than anandamide in mouse brain homogenates and is primarily metabolized by epoxide hydrolase. Combined, our results suggest that epoxidation of anandamide by P450s to form 5,6-EET-EA represents an endocannabinoid bioactivation pathway in the context of immune cell function.
Figures


Similar articles
-
Anandamide metabolism by human liver and kidney microsomal cytochrome p450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides.J Pharmacol Exp Ther. 2007 May;321(2):590-7. doi: 10.1124/jpet.107.119321. Epub 2007 Feb 1. J Pharmacol Exp Ther. 2007. PMID: 17272674
-
Anandamide oxidation by wild-type and polymorphically expressed CYP2B6 and CYP2D6.Drug Metab Dispos. 2011 May;39(5):782-8. doi: 10.1124/dmd.110.036707. Epub 2011 Feb 2. Drug Metab Dispos. 2011. PMID: 21289075 Free PMC article.
-
The endocannabinoid anandamide is a substrate for the human polymorphic cytochrome P450 2D6.J Pharmacol Exp Ther. 2008 Nov;327(2):538-45. doi: 10.1124/jpet.108.141796. Epub 2008 Aug 12. J Pharmacol Exp Ther. 2008. PMID: 18698000 Free PMC article.
-
Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications.Pharmacol Rev. 2010 Mar;62(1):136-54. doi: 10.1124/pr.109.001081. Epub 2010 Feb 4. Pharmacol Rev. 2010. PMID: 20133390 Free PMC article. Review.
-
Why do cannabinoid receptors have more than one endogenous ligand?Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3216-28. doi: 10.1098/rstb.2011.0382. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108541 Free PMC article. Review.
Cited by
-
Lipoxygenase-mediated oxidation of polyunsaturated N-acylethanolamines in Arabidopsis.J Biol Chem. 2011 Apr 29;286(17):15205-14. doi: 10.1074/jbc.M110.217588. Epub 2011 Mar 3. J Biol Chem. 2011. PMID: 21372125 Free PMC article.
-
Cross-talk of cannabinoid and endocannabinoid metabolism is mediated via human cardiac CYP2J2.J Inorg Biochem. 2018 Jul;184:88-99. doi: 10.1016/j.jinorgbio.2018.03.016. Epub 2018 Apr 7. J Inorg Biochem. 2018. PMID: 29689453 Free PMC article.
-
Effects of a commonly occurring genetic polymorphism of human CYP3A4 (I118V) on the metabolism of anandamide.Drug Metab Dispos. 2010 Nov;38(11):2075-82. doi: 10.1124/dmd.110.033712. Epub 2010 Aug 11. Drug Metab Dispos. 2010. PMID: 20702771 Free PMC article.
-
Detoxification Cytochrome P450s (CYPs) in Families 1-3 Produce Functional Oxylipins from Polyunsaturated Fatty Acids.Cells. 2022 Dec 24;12(1):82. doi: 10.3390/cells12010082. Cells. 2022. PMID: 36611876 Free PMC article. Review.
-
Design and Potency of Dual Soluble Epoxide Hydrolase/Fatty Acid Amide Hydrolase Inhibitors.ACS Omega. 2018 Oct 31;3(10):14076-14086. doi: 10.1021/acsomega.8b01625. Epub 2018 Oct 25. ACS Omega. 2018. PMID: 30411058 Free PMC article.
References
-
- Block ML, Zecca L, and Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8 57-69. - PubMed
-
- Bocchini V, Mazzolla R, Barluzzi R, Blasi E, Sick P, and Kettenmann H (1992) An immortalized cell line expresses properties of activated microglial cells. J Neurosci Res 31 616-621. - PubMed
-
- Bornheim LM, Kim KY, Chen B, and Correia MA (1995) Microsomal cytochrome P450-mediated liver and brain anandamide metabolism. Biochem Pharmacol 50 677-686. - PubMed
-
- Brown HS, Chadwick A, and Houston JB (2007) Use of isolated hepatocyte preparations for cytochrome P450 inhibition studies: comparison with microsomes for Ki determination [published erratum appears in: Drug Metab Dispos 36:203, 2008]. Drug Metab Dispos 35 2119-2126. - PubMed
-
- Cabral GA and Marciano-Cabral F (2005) Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. J Leukoc Biol 78 1192-1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

