Translocation or just location? Pseudopodia affect fluorescent signals
- PMID: 19171754
- PMCID: PMC2654297
- DOI: 10.1083/jcb.200806047
Translocation or just location? Pseudopodia affect fluorescent signals
Abstract
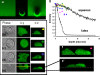
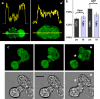
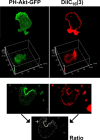
The use of fluorescent probes is one of the most powerful techniques for gaining spatial and temporal knowledge of dynamic events within living cells. Localized increases in the signal from cytosolic fluorescent protein constructs, for example, are frequently used as evidence for translocation of proteins to specific sites within the cell. However, differences in optical and geometrical properties of cytoplasm can influence the recorded intensity of the probe signal. Pseudopodia are especially problematic because their cytoplasmic properties can cause abrupt increases in fluorescent signal of both GFP and fluorescein. Investigators should therefore be cautious when interpreting fluorescence changes within a cell, as these can result from either translocation of the probe or changes in the optical properties of the milieu surrounding the probe.
Figures





References
-
- Aizawa H., Fukui Y., Yahara I. 1997. Live dynamics of Dictyostelium cofilin suggests a role in remodeling actin latticework into bundles.J. Cell Sci. 110:2333–2344 - PubMed
-
- Bessis M. 1973. Living Blood Cells and their Ultrastructure Springer-Verlag, Berlin. New York: 298–323
-
- Brundage R.A., Fogarty K.E., Tuft R.A., Fay F.S. 1991. Ca2+ gradients underlying polarization and chemotaxis of eosinophils.Science. 254:703–705 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

