TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria
- PMID: 19171755
- PMCID: PMC2654305
- DOI: 10.1083/jcb.200808080
TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria
Abstract
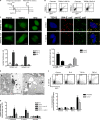
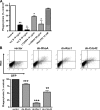
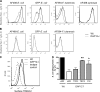
Phagocytosis, which is essential for the immune response to pathogens, is initiated by specific interactions between pathogens and cell surface receptors expressed by phagocytes. This study identifies triggering receptor expressed on myeloid cells 2 (TREM-2) and its signaling counterpart DAP12 as a molecular complex that promotes phagocytosis of bacteria. Expression of TREM-2-DAP12 enables nonphagocytic Chinese hamster ovary cells to internalize bacteria. This function depends on actin cytoskeleton dynamics and the activity of the small guanosine triphosphatases Rac and Cdc42. Internalization also requires src kinase activity and tyrosine phosphorylation. In bone marrow-derived macrophages, phagocytosis is decreased in the absence of DAP12 and can be restored by expression of TREM-2-DAP12. Depletion of TREM-2 inhibits both binding and uptake of bacteria. Finally, TREM-2-dependent phagocytosis is impaired in Syk-deficient macrophages. This study highlights a novel role for TREM-2-DAP12 in the immune response to bacterial pathogens.
Figures


References
-
- Aderem A., Underhill D.M. 1999. Mechanisms of phagocytosis in macrophages.Annu. Rev. Immunol. 17:593–623 - PubMed
-
- Andrews S., Stephens L.R., Hawkins P.T. 2007. PI3K class IB pathway in neutrophils.Sci. STKE. doi:10.1126/stke.4072007cm3 - DOI - PubMed
-
- Bakker A.B., Hoek R.M., Cerwenka A., Blom B., Lucian L., McNeil T., Murray R., Phillips L.H., Sedgwick J.D., Lanier L.L. 2000. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming.Immunity. 13:345–353 - PubMed
-
- Berton G., Mocsai A., Lowell C.A. 2005. Src and Syk kinases: key regulators of phagocytic cell activation.Trends Immunol. 26:208–214 - PubMed
-
- Brown E.J. 1995. Phagocytosis.Bioessays. 17:109–117 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

