Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas
- PMID: 19171757
- PMCID: PMC2654302
- DOI: 10.1083/jcb.200807019
Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas
Abstract
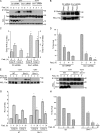
Reactive oxygen species (ROS) increase ligation of Fas (CD95), a receptor important for regulation of programmed cell death. Glutathionylation of reactive cysteines represents an oxidative modification that can be reversed by glutaredoxins (Grxs). The goal of this study was to determine whether Fas is redox regulated under physiological conditions. In this study, we demonstrate that stimulation with Fas ligand (FasL) induces S-glutathionylation of Fas at cysteine 294 independently of nicotinamide adenine dinucleotide phosphate reduced oxidase-induced ROS. Instead, Fas is S-glutathionylated after caspase-dependent degradation of Grx1, increasing subsequent caspase activation and apoptosis. Conversely, overexpression of Grx1 attenuates S-glutathionylation of Fas and partially protects against FasL-induced apoptosis. Redox-mediated Fas modification promotes its aggregation and recruitment into lipid rafts and enhances binding of FasL. As a result, death-inducing signaling complex formation is also increased, and subsequent activation of caspase-8 and -3 is augmented. These results define a novel redox-based mechanism to propagate Fas-dependent apoptosis.
Figures


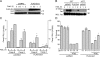


References
-
- Daily D., Vlamis-Gardikas A., Offen D., Mittelman L., Melamed E., Holmgren A., Barzilai A. 2001. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and jun n-terminal kinase pathways.J. Biol. Chem. 276:21618–21626 - PubMed
-
- Earnshaw W.C., Martins L.M., Kaufmann S.H. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis.Annu. Rev. Biochem. 68:383–424 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

