Decremental response to high-frequency trains of acetylcholine pulses but unaltered fractional Ca2+ currents in a panel of "slow-channel syndrome" nicotinic receptor mutants
- PMID: 19171769
- PMCID: PMC2638206
- DOI: 10.1085/jgp.200810089
Decremental response to high-frequency trains of acetylcholine pulses but unaltered fractional Ca2+ currents in a panel of "slow-channel syndrome" nicotinic receptor mutants
Abstract
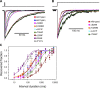
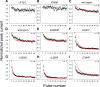
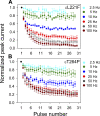
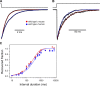
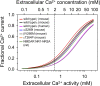
The slow-channel congenital myasthenic syndrome (SCCMS) is a disorder of the neuromuscular junction caused by gain-of-function mutations to the muscle nicotinic acetylcholine (ACh) receptor (AChR). Although it is clear that the slower deactivation time course of the ACh-elicited currents plays a central role in the etiology of this disease, it has been suggested that other abnormal properties of these mutant receptors may also be critical in this respect. We characterized the kinetics of a panel of five SCCMS AChRs (alphaS269I, betaV266M, epsilonL221F, epsilonT264P, and epsilonL269F) at the ensemble level in rapidly perfused outside-out patches. We found that, for all of these mutants, the peak-current amplitude decreases along trains of nearly saturating ACh pulses delivered at physiologically relevant frequencies in a manner that is consistent with enhanced entry into desensitization during the prolonged deactivation phase. This suggests that the increasingly reduced availability of activatable AChRs upon repetitive stimulation may well contribute to the fatigability and weakness of skeletal muscle that characterize this disease. Also, these results emphasize the importance of explicitly accounting for entry into desensitization as one of the pathways for burst termination, if meaningful mechanistic insight is to be inferred from the study of the effect of these naturally occurring mutations on channel function. Applying a novel single-channel-based approach to estimate the contribution of Ca(2+) to the total cation currents, we also found that none of these mutants affects the Ca(2+)-conduction properties of the AChR to an extent that seems to be of physiological importance. Our estimate of the Ca(2+)-carried component of the total (inward) conductance of wild-type and SCCMS AChRs in the presence of 150 mM Na(+), 1.8 mM Ca(2+), and 1.7 mM Mg(2+) on the extracellular side of cell-attached patches turned out be in the 5.0-9.4 pS range, representing a fractional Ca(2+) current of approximately 14%, on average. Remarkably, these values are nearly identical to those we estimated for the NR1-NR2A N-methyl-d-aspartate receptor (NMDAR), which has generally been considered to be the main neurotransmitter-gated pathway of Ca(2+) entry into the cell. Our estimate of the rat NMDAR Ca(2+) conductance (using the same single-channel approach as for the AChR but in the nominal absence of extracellular Mg(2+)) was 7.9 pS, corresponding to a fractional Ca(2+) current of 13%.
Figures



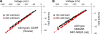




Comment in
-
About a new method to measure fractional Ca2+ currents through ligand-gated ion channels.J Gen Physiol. 2009 Sep;134(3):259-61; author reply 263-5. doi: 10.1085/jgp.200910222. J Gen Physiol. 2009. PMID: 19720963 Free PMC article. No abstract available.
References
-
- Barbour B., Häusser M. 1997. Intersynaptic diffusion of neurotransmitter.Trends Neurosci. 20:377–384 - PubMed
-
- Barry P.H. 1994. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements.J. Neurosci. Methods. 51:107–116 - PubMed
-
- Barry P.H., Lynch J.W. 1991. Liquid junction potentials and small cell effects in patch-clamp analysis.J. Membr. Biol. 121:101–117 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

