Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model
- PMID: 19171883
- PMCID: PMC2650174
- DOI: 10.1073/pnas.0810902106
Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model
Abstract
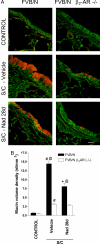
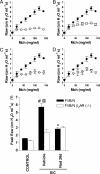
Chronic regular use of beta(2)-adrenoceptor (beta(2)-AR) agonists in asthma is associated with a loss of disease control and increased risk of death. Conversely, we have found that administration of beta(2)-AR inverse agonists results in attenuation of the asthma phenotype in an allergen-driven murine model. Besides antagonizing agonist-induced signaling and reducing signaling by empty receptors, beta-AR inverse agonists can also activate signaling by novel pathways. To determine the mechanism of the beta-AR inverse agonists, we compared the asthma phenotype in beta(2)-AR-null and wild-type mice. Antigen challenge of beta(2)-AR-null mice produced results similar to what was observed with chronic beta(2)-AR inverse agonist treatment, namely, reductions in mucous metaplasia, airway hyperresponsiveness (AHR), and inflammatory cells in the lungs. These results indicate that the effects of beta(2)-AR inverse agonists are caused by inhibition of beta(2)-AR signaling rather than by the induction of novel signaling pathways. Chronic administration of alprenolol, a beta-blocker without inverse agonist properties, did not attenuate the asthma phenotype, suggesting that it is signaling by empty receptors, rather than agonist-induced beta(2)-AR signaling, that supports the asthma phenotype. In conclusion, our results demonstrate that, in a murine model of asthma, beta(2)-AR signaling is required for the full development of three cardinal features of asthma: mucous metaplasia, AHR, and the presence of inflammatory cells in the lungs.
Conflict of interest statement
Conflict of interest statement: R.A.B. is a scientific founder and shareholder of Inverseon, Inc. S.P. is a shareholder of Inverseon, Inc.
Figures


Comment in
-
Agonizing over agonism: should asthmatics turn their beta-receptors on or off?Proc Natl Acad Sci U S A. 2009 Feb 17;106(7):2095-6. doi: 10.1073/pnas.0812935106. Epub 2009 Feb 11. Proc Natl Acad Sci U S A. 2009. PMID: 19211783 Free PMC article. No abstract available.
References
-
- Lipworth BJ. Airway subsensitivity with long-acting β2-agonists: Is there cause for concern? Drug Saf. 1997;16:295–308. - PubMed
-
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: Effect of long-acting β-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144:904–912. - PubMed
-
- Abramson MJ, Walters J, Walters EH. Adverse effects of β-agonists: Are they clinically relevant? Am J Respir Med. 2003;2:287–297. - PubMed
-
- Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet. 1993;342:833–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

