Expression profiling during ocular development identifies 2 Nlz genes with a critical role in optic fissure closure
- PMID: 19171890
- PMCID: PMC2631080
- DOI: 10.1073/pnas.0812017106
Expression profiling during ocular development identifies 2 Nlz genes with a critical role in optic fissure closure
Abstract
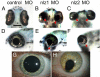
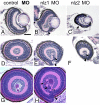
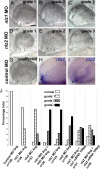
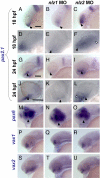
The gene networks underlying closure of the optic fissure during vertebrate eye development are poorly understood. Here, we profile global gene expression during optic fissure closure using laser capture microdissected (LCM) tissue from the margins of the fissure. From these data, we identify a unique role for the C(2)H(2) zinc finger proteins Nlz1 and Nlz2 in normal fissure closure. Gene knockdown of nlz1 and/or nlz2 in zebrafish leads to a failure of the optic fissure to close, a phenotype which closely resembles that seen in human uveal coloboma. We also identify misregulation of pax2 in the developing eye of morphant fish, suggesting that Nlz1 and Nlz2 act upstream of the Pax2 pathway in directing proper closure of the optic fissure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- O'Rahilly The early development of the eye in staged human embryos. Contrib Embryol. 1966;XXXVIII(25–263):1–45.
-
- Bermejo E, Martinez-Frias ML. Congenital eye malformations: clinical-epidemiological analysis of 1,124,654 consecutive births in Spain. Am J Med Genet. 1998;75(5):497–504. - PubMed
-
- Porges Y, et al. Hereditary microphthalmia with colobomatous cyst. Am J Ophthalmol. 1992;114(1):30–34. - PubMed
-
- Traboulsi E. Colobomatous micropthalmia, anophthalmia, and associated malformation syndromes. In: Traboulsi E, editor. Genetic Diseases of the Eye. New York: Oxford Univ Press; 1999. pp. 51–80.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

