The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications
- PMID: 19171928
- PMCID: PMC3170409
- DOI: 10.1177/0192623308327119
The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications
Abstract
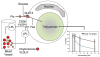
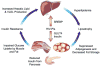
Metabolic complications associated with HIV infection and treatment frequently present as a relative lack of peripheral adipose tissue associated with dyslipidemia and insulin resistance. In this review we explain the connection between abnormalities of intermediary metabolism, observed either in vitro or in vivo, and this group of metabolic effects. We review molecular mechanisms by which the HIV protease inhibitor (PI) class of drugs may affect the normal stimulatory effect of insulin on glucose and fat storage. We then propose that both chronic inflammation from HIV infection and treatment with some drugs in this class trigger cellular homeostatic stress responses with adverse effects on intermediary metabolism. The physiologic outcome is such that total adipocyte storage capacity is decreased, and the remaining adipocytes resist further fat storage. The excess circulating and dietary lipid metabolites, normally "absorbed" by adipose tissue, are deposited ectopically in lean (muscle and liver) tissue, where they impair insulin action. This process leads to a pathologic cycle of lipotoxicity and lipoatrophy and a clinical phenotype of body fat distribution with elevated waist-to-hip ratio similar to the metabolic syndrome.
Figures



References
-
- Arranz Caso JA, Lopez JC, Santos I, Estrada V, Castilla V, Sanz J, Molina JP, Fernandez Guerrero M, Gorgolas M. A randomized controlled trial investigating the efficacy and safety of switching from a protease inhibitor to nevirapine in patients with undetectable viral load. HIV Med. 2005;6:353–59. - PubMed
-
- Barbaro G. Visceral fat as target of highly active antiretroviral therapy-associated metabolic syndrome. Curr Pharm Des. 2007;13:2208–13. - PubMed
-
- Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, Korner T, Stoll M, Schmidt RE. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–70. - PubMed
-
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

