Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation
- PMID: 19172146
- PMCID: PMC2658727
- DOI: 10.1038/nchembio.147
Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation
Abstract
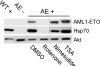
It has been proposed that inhibitors of an oncogene's effects on multipotent hematopoietic progenitor cell differentiation may change the properties of the leukemic stem cells and complement the clinical use of cytotoxic drugs. Using zebrafish, we developed a robust in vivo hematopoietic differentiation assay that reflects the activity of the oncogene AML1-ETO. Screening for modifiers of AML1-ETO-mediated hematopoietic dysregulation uncovered unexpected roles of COX-2- and beta-catenin-dependent pathways in AML1-ETO function. This approach may open doors for developing therapeutics targeting oncogene function within leukemic stem cells.
Figures
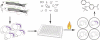


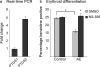



Similar articles
-
AML1-ETO reprograms hematopoietic cell fate by downregulating scl expression.Development. 2008 Jan;135(2):401-10. doi: 10.1242/dev.008904. Development. 2008. PMID: 18156164
-
AML1-ETO mediates hematopoietic self-renewal and leukemogenesis through a COX/β-catenin signaling pathway.Blood. 2013 Jun 13;121(24):4906-16. doi: 10.1182/blood-2012-08-447763. Epub 2013 May 3. Blood. 2013. PMID: 23645839 Free PMC article.
-
Zebrafish small molecule screen in reprogramming/cell fate modulation.Methods Mol Biol. 2010;636:317-27. doi: 10.1007/978-1-60761-691-7_20. Methods Mol Biol. 2010. PMID: 20336532 Free PMC article.
-
Oncogenic pathways of AML1-ETO in acute myeloid leukemia: multifaceted manipulation of marrow maturation.Cancer Lett. 2007 Jun 28;251(2):179-86. doi: 10.1016/j.canlet.2006.10.010. Epub 2006 Nov 27. Cancer Lett. 2007. PMID: 17125917 Free PMC article. Review.
-
Effects of the leukemia-associated AML1-ETO protein on hematopoietic stem and progenitor cells.Oncogene. 2004 May 24;23(24):4249-54. doi: 10.1038/sj.onc.1207673. Oncogene. 2004. PMID: 15156180 Review.
Cited by
-
From phenotype to mechanism after zebrafish small molecule screens.Drug Discov Today Dis Models. 2013 Spring;10(1):e51-e55. doi: 10.1016/j.ddmod.2012.02.002. Drug Discov Today Dis Models. 2013. PMID: 26146505 Free PMC article.
-
Identification of mundoserone by zebrafish in vivo screening as a natural product with anti-angiogenic activity.Exp Ther Med. 2018 Dec;16(6):4562-4568. doi: 10.3892/etm.2018.6748. Epub 2018 Sep 17. Exp Ther Med. 2018. PMID: 30542405 Free PMC article.
-
Zebrafish and Medaka: new model organisms for modern biomedical research.J Biomed Sci. 2016 Jan 28;23:19. doi: 10.1186/s12929-016-0236-5. J Biomed Sci. 2016. PMID: 26822757 Free PMC article. Review.
-
Development of an In Vitro Assay to Quantitate Hematopoietic Stem and Progenitor Cells (HSPCs) in Developing Zebrafish Embryos.J Vis Exp. 2017 Nov 30;(129):56836. doi: 10.3791/56836. J Vis Exp. 2017. PMID: 29286381 Free PMC article.
-
RUNX1 and RUNX1-ETO: roles in hematopoiesis and leukemogenesis.Front Biosci (Landmark Ed). 2012 Jan 1;17(3):1120-39. doi: 10.2741/3977. Front Biosci (Landmark Ed). 2012. PMID: 22201794 Free PMC article. Review.
References
-
- Redaelli A, Botteman MF, Stephens JM, Brandt S, Pashos CL. Economic burden of acute myeloid leukemia: a literature review. Cancer Treat Rev. 2004;30:237–247. - PubMed
-
- Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood. 2003;101:3142–3149. - PubMed
-
- Terpstra W, et al. Fluorouracil selectively spares acute myeloid leukemia cells with long-term growth abilities in immunodeficient mice and in culture. Blood. 1996;88:1944–1950. - PubMed
-
- Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. - PubMed
-
- Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003;3:89–101. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/56459045
- PubChem-Substance/56459046
- PubChem-Substance/56459047
- PubChem-Substance/56459048
- PubChem-Substance/56459049
- PubChem-Substance/56459050
- PubChem-Substance/56459051
- PubChem-Substance/56459052
- PubChem-Substance/56459053
- PubChem-Substance/56459054
- PubChem-Substance/56459055
- PubChem-Substance/56459056
- PubChem-Substance/56459057
- PubChem-Substance/56459058
- PubChem-Substance/56459059
- PubChem-Substance/56459060
- PubChem-Substance/56459061
- PubChem-Substance/56459062
- PubChem-Substance/56459063
- PubChem-Substance/56459064
- PubChem-Substance/56459065
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

