The importance of pH in regulating the function of the Fasciola hepatica cathepsin L1 cysteine protease
- PMID: 19172172
- PMCID: PMC2621350
- DOI: 10.1371/journal.pntd.0000369
The importance of pH in regulating the function of the Fasciola hepatica cathepsin L1 cysteine protease
Abstract
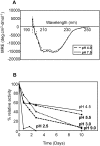
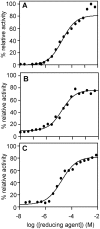
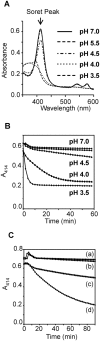
The helminth parasite Fasciola hepatica secretes cathepsin L cysteine proteases to invade its host, migrate through tissues and digest haemoglobin, its main source of amino acids. Here we investigated the importance of pH in regulating the activity and functions of the major cathepsin L protease FheCL1. The slightly acidic pH of the parasite gut facilitates the auto-catalytic activation of FheCL1 from its inactive proFheCL1 zymogen; this process was approximately 40-fold faster at pH 4.5 than at pH 7.0. Active mature FheCL1 is very stable at acidic and neutral conditions (the enzyme retained approximately 45% activity when incubated at 37 degrees C and pH 4.5 for 10 days) and displayed a broad pH range for activity peptide substrates and the protein ovalbumin, peaking between pH 5.5 and pH 7.0. This pH profile likely reflects the need for FheCL1 to function both in the parasite gut and in the host tissues. FheCL1, however, could not cleave its natural substrate Hb in the pH range pH 5.5 and pH 7.0; digestion occurred only at pH</=4.5, which coincided with pH-induced dissociation of the Hb tetramer. Our studies indicate that the acidic pH of the parasite relaxes the Hb structure, making it susceptible to proteolysis by FheCL1. This process is enhanced by glutathione (GSH), the main reducing agent contained in red blood cells. Using mass spectrometry, we show that FheCL1 can degrade Hb to small peptides, predominantly of 4-14 residues, but cannot release free amino acids. Therefore, we suggest that Hb degradation is not completed in the gut lumen but that the resulting peptides are absorbed by the gut epithelial cells for further processing by intracellular di- and amino-peptidases to free amino acids that are distributed through the parasite tissue for protein anabolism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Andrews SJ. In: Fasciolosis. Dalton JP, editor. Oxford: CABI; 1999. pp. 1–29.
-
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. - PubMed
-
- MacManus DP, Dalton JD. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology. 2006;133:S43–61. - PubMed
-
- McGonigle L, Mousley A, Marks NJ, Brennan GP, Dalton JP, et al. The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. Int J Parasitol. 2008;38:149–155. - PubMed
-
- Mulcahy G, O'Connor F, Clery D, Hogan SF, Dowd AJ, et al. Immune responses of cattle to experimental anti-Fasciola hepatica vaccines. Res Vet Sci. 1999;67:27–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

