Is vitamin E toxic to neuron cells?
- PMID: 19172392
- PMCID: PMC11506026
- DOI: 10.1007/s10571-008-9340-8
Is vitamin E toxic to neuron cells?
Abstract
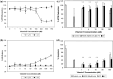
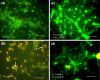
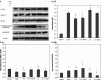
Besides acting as potent free radical scavengers, tocopherols and tocotrienols have been known to have non-antioxidant properties such as the involvement of alpha-tocopherol (alphaT) in PKC pathway and the anti-cancer properties of gamma-tocotrienol (gammaT3). This study aims to elucidate whether protective effects shown by alphaT and gammaT3 in H(2)O(2)-induced neuron cultures have anti-apoptotic or pro-apoptotic tendency toward the initiation of neuronal apoptosis. H(2)O(2) is used to induce apoptosis in primary cerebellar neuron cultures which is attenuated by pretreatment of alphaT or gammaT3 at concentrations < or =10 microM. Similar to our previous work, gammaT3 was found to be neurotoxic at concentrations > or =100 microM, whereas alphaT showed no neurotoxicity. Cellular uptake of gammaT3 was higher than that of alphaT. Treating cells simultaneously with either gammaT3 or alphaT and with then H(2)O(2) led to higher expression of Bax and Bcl-2 than in neurons exposed to H(2)O(2) alone. Analysis of Bcl-2/Bax ratio as 'survival index' showed that both pretreatment of gammaT3 and alphaT followed by H(2)O(2) increase the 'survival index' of Bcl-2/Bax ratio compared to H(2)O(2)-treated cells, while treatment of gammaT3 alone decrease the ratio compared to unchanged Bcl2/Bax ratio of similar treatment with alphaT alone. Similar treatment of gammaT3 decreased p53 expression and activates p38 MAPK phosphorylation, whereas alphaT did not alter its expression compared to H(2)O(2)-treated cells. Treating neurons with only gammaT3 or alphaT increased the expression of Bax, Bcl-2, p53, and p38 MAPK compared to control with gammaT3 exerting stronger expression for proteins involved than alphaT. In conclusion, low doses of gammaT3 and alphaT confer neuroprotection to H(2)O(2)-treated neurons via their antioxidant mechanism but gammaT3 has stronger pro-apoptosis tendency than alphaT by activating molecules involved in the neuronal apoptotic pathway in the absence of H(2)O(2).
Figures



Similar articles
-
γ-Tocotrienol does not substantially protect DS neurons from hydrogen peroxide-induced oxidative injury.Nutr Metab (Lond). 2012 Jan 5;9:1. doi: 10.1186/1743-7075-9-1. Nutr Metab (Lond). 2012. PMID: 22217149 Free PMC article.
-
Vitamin E protected cultured cortical neurons from oxidative stress-induced cell death through the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase.J Neurochem. 2006 May;97(4):1191-202. doi: 10.1111/j.1471-4159.2006.03827.x. J Neurochem. 2006. PMID: 16686696
-
Distinct roles of different forms of vitamin E in DHA-induced apoptosis in triple-negative breast cancer cells.Mol Nutr Food Res. 2012 Jun;56(6):923-34. doi: 10.1002/mnfr.201200027. Mol Nutr Food Res. 2012. PMID: 22707267
-
Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy.Adv Nutr. 2017 Nov 15;8(6):850-867. doi: 10.3945/an.117.016329. Print 2017 Nov. Adv Nutr. 2017. PMID: 29141970 Free PMC article. Review.
-
Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy.Free Radic Biol Med. 2014 Jul;72:76-90. doi: 10.1016/j.freeradbiomed.2014.03.035. Epub 2014 Apr 3. Free Radic Biol Med. 2014. PMID: 24704972 Free PMC article. Review.
Cited by
-
High concentration of vitamin E decreases thermosensation and thermotaxis learning and the underlying mechanisms in the nematode Caenorhabditis elegans.PLoS One. 2013 Aug 12;8(8):e71180. doi: 10.1371/journal.pone.0071180. eCollection 2013. PLoS One. 2013. PMID: 23951104 Free PMC article.
-
The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes.Bosn J Basic Med Sci. 2014 Nov 16;14(4):195-204. doi: 10.17305/bjbms.2014.4.91. Bosn J Basic Med Sci. 2014. PMID: 25428670 Free PMC article.
-
The difference in the cellular uptake of tocopherol and tocotrienol is influenced by their affinities to albumin.Sci Rep. 2023 May 6;13(1):7392. doi: 10.1038/s41598-023-34584-z. Sci Rep. 2023. PMID: 37149706 Free PMC article.
-
γ-Tocotrienol protects against mitochondrial dysfunction and renal cell death.J Pharmacol Exp Ther. 2012 Feb;340(2):330-8. doi: 10.1124/jpet.111.186882. Epub 2011 Oct 31. J Pharmacol Exp Ther. 2012. PMID: 22040679 Free PMC article.
-
γ-Tocotrienol does not substantially protect DS neurons from hydrogen peroxide-induced oxidative injury.Nutr Metab (Lond). 2012 Jan 5;9:1. doi: 10.1186/1743-7075-9-1. Nutr Metab (Lond). 2012. PMID: 22217149 Free PMC article.
References
-
- Agarwal MK, Agarwal ML, Athar M, Gupta S (2004) Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl-2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle 3:1–7 - PubMed
-
- Alvarado C, Alvarez P, Puerto M, Gausserès N, Jiménez L, De la Fuente M (2006) Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition 22:767–777. doi:10.1016/j.nut.2006.05.007 - PubMed
-
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science 277:370–372. doi:10.1126/science.277.5324.370 - PubMed
-
- Azzi A, Stocker A (2000) Vitamin E: non-antioxidant roles. Prog Lipid Res 39:231–255. doi:10.1016/S0163-7827(00)00006-0 - PubMed
-
- Banudevi S, Krishnamoorthy G, Venkataraman P, Vignesh C, Aruldhas MM, Arunakaran J (2006) Role of alpha-tocopherol on antioxidant status in liver, lung and kidney of PCB exposed male albino rats. Food Chem Toxicol 44:2040–2046. doi:10.1016/j.fct.2006.07.017 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

