Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls
- PMID: 19172649
- PMCID: PMC2733926
- DOI: 10.1002/hbm.20708
Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls
Abstract
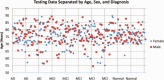
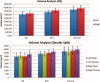
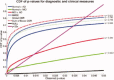
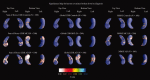
We used a new method we developed for automated hippocampal segmentation, called the auto context model, to analyze brain MRI scans of 400 subjects from the Alzheimer's disease neuroimaging initiative. After training the classifier on 21 hand-labeled expert segmentations, we created binary maps of the hippocampus for three age- and sex-matched groups: 100 subjects with Alzheimer's disease (AD), 200 with mild cognitive impairment (MCI) and 100 elderly controls (mean age: 75.84; SD: 6.64). Hippocampal traces were converted to parametric surface meshes and a radial atrophy mapping technique was used to compute average surface models and local statistics of atrophy. Surface-based statistical maps visualized links between regional atrophy and diagnosis (MCI versus controls: P = 0.008; MCI versus AD: P = 0.001), mini-mental state exam (MMSE) scores, and global and sum-of-boxes clinical dementia rating scores (CDR; all P < 0.0001, corrected). Right but not left hippocampal atrophy was associated with geriatric depression scores (P = 0.004, corrected); hippocampal atrophy was not associated with subsequent decline in MMSE and CDR scores, educational level, ApoE genotype, systolic or diastolic blood pressure measures, or homocysteine. We gradually reduced sample sizes and used false discovery rate curves to examine the method's power to detect associations with diagnosis and cognition in smaller samples. Forty subjects were sufficient to discriminate AD from normal and correlate atrophy with CDR scores; 104, 200, and 304 subjects, respectively, were required to correlate MMSE with atrophy, to distinguish MCI from normal, and MCI from AD.
2009 Wiley-Liss, Inc.
Figures


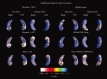
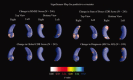

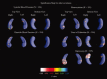
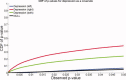

References
-
- Apostolova LG,Dinov ID,Dutton RA,Hayashi KM,Toga AW,Cummings JL,Thompson PM ( 2006a): 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain 129(Pt 11): 2867–2873. - PubMed
-
- Apostolova LG,Dutton RA,Dinov ID,Hayashi KM,Toga AW,Cummings JL,Thompson PM ( 2006b): Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol 63: 693–699. - PubMed
-
- Apostolova LG,Akopyan GG,Partiali N,Steiner CA,Dutton RA,Hayashi KM,Dinov ID,Toga AW,Cummings JL,Thompson PM ( 2007): Structural correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord 24: 91–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

