Formation and function of the lytic NK-cell immunological synapse
- PMID: 19172692
- PMCID: PMC2772177
- DOI: 10.1038/nri2381
Formation and function of the lytic NK-cell immunological synapse
Abstract
The natural killer (NK)-cell immunological synapse is the dynamic interface formed between an NK cell and its target cell. Formation of the NK-cell immunological synapse involves several distinct stages, from the initiation of contact with a target cell to the directed delivery of lytic-granule contents for target-cell lysis. Progression through the individual stages is regulated, and this tight regulation underlies the precision with which NK cells select and kill susceptible target cells (including virally infected cells and cancerous cells) that they encounter during their routine surveillance of the body.
Figures
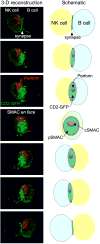

References
-
- Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. - PubMed
-
- Wulfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci U S A. 2003;100:7767–72. Introduction of steps in NK cell immunological synapse formation centered around the cytoskeleton (see also #12) with critical comparisons to T cells underscoring differences. - PMC - PubMed
-
- Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. - PubMed
-
- Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends Immunol. 2004;25:323–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

