Antitumor activity of T cells generated from lymph nodes draining the SEA-expressing murine B16 melanoma and secondarily activated with dendritic cells
- PMID: 19173035
- PMCID: PMC2631223
- DOI: 10.7150/ijbs.5.135
Antitumor activity of T cells generated from lymph nodes draining the SEA-expressing murine B16 melanoma and secondarily activated with dendritic cells
Abstract
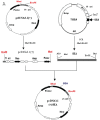
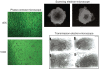
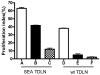
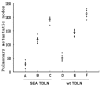
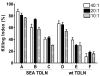
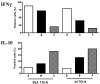
The successful use of tumor-draining lymph nodes (TDLN) as a source of effector cells for cancer immunotherapy depends largely on the immunogenicity of the tumor drained by the lymph nodes as well as the methods for secondary in vitro T cell activation and expansion. We transferred the bacterial superantigen staphylococcal enterotoxin A (SEA) gene into B16 murine melanoma tumor cells, and used them to induce TDLN (SEA TDLN) in syngeneic hosts. Wild-type (wt) TDLN induced by parental B16 tumor was used as a control. In vitro, SEA TDLN cells proliferated more vigorously, produced more IFN gamma and demonstrated higher CTL activity than wt TDLN cells when activated with anti-CD3/anti-CD28/IL-2. In vivo, SEA TDLN cells mediated tumor eradication more effectively than similarly activated wt TDLN cells (p<0.01). Furthermore, use of dendritic cells (DC) plus tumor antigen in vitro in addition to anti-CD3/anti-CD28/IL-2 stimulation further amplified the immune function and therapeutic efficacy of SEA TDLN cells. DC-stimulated SEA TDLN cells eliminated nearly 90% of the pulmonary metastasis in mice bearing established B16 melanoma micrometastases. These results indicate that enforced expression of superantigen SEA in poorly immunogenic tumor cells can enhance their immunogenicity as a vaccine in vivo. The combined use of genetically modified tumor cells as vaccine to induce TDLN followed by secondary stimulation using antigen-presenting cells and tumor antigen in a sequential immunization/activation procedure may represent a unique method to generate more potent effector T cells for adoptive immunotherapy of cancer.
Keywords: Adoptive therapy; B16 melanoma; Staphylococcal enterotoxin A (SEA); T lymphocyte; dendritic cells (DC).
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures


Similar articles
-
Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells.J Immunol. 2005 Aug 1;175(3):1424-32. doi: 10.4049/jimmunol.175.3.1424. J Immunol. 2005. PMID: 16034078
-
Adoptive immunotherapy of murine intracerebral tumors with anti-CD3/interleukin-2-activated tumor-draining lymph node cells.J Immunother Emphasis Tumor Immunol. 1994 May;15(4):242-50. doi: 10.1097/00002371-199405000-00002. J Immunother Emphasis Tumor Immunol. 1994. PMID: 8061896
-
Successful adoptive immunotherapy of murine poorly immunogenic tumor with specific effector cells generated from gene-modified tumor-primed lymph node cells.J Immunol. 1999 Mar 15;162(6):3574-82. J Immunol. 1999. PMID: 10092816
-
Immunotherapy Goes Local: The Central Role of Lymph Nodes in Driving Tumor Infiltration and Efficacy.Front Immunol. 2021 Mar 1;12:643291. doi: 10.3389/fimmu.2021.643291. eCollection 2021. Front Immunol. 2021. PMID: 33732264 Free PMC article. Review.
-
Immune checkpoints targeting dendritic cells for antibody-based modulation in cancer.Int Rev Cell Mol Biol. 2024;382:145-179. doi: 10.1016/bs.ircmb.2023.07.006. Epub 2023 Aug 12. Int Rev Cell Mol Biol. 2024. PMID: 38225102 Review.
Cited by
-
Normalization of tumor microenvironment by neem leaf glycoprotein potentiates effector T cell functions and therapeutically intervenes in the growth of mouse sarcoma.PLoS One. 2013 Jun 13;8(6):e66501. doi: 10.1371/journal.pone.0066501. Print 2013. PLoS One. 2013. PMID: 23785504 Free PMC article.
-
RSK2 phosphorylates T-bet to attenuate colon cancer metastasis and growth.Proc Natl Acad Sci U S A. 2017 Nov 28;114(48):12791-12796. doi: 10.1073/pnas.1710756114. Epub 2017 Nov 13. Proc Natl Acad Sci U S A. 2017. PMID: 29133416 Free PMC article.
-
The intersection of immune-directed and molecularly targeted therapy in advanced melanoma: where we have been, are, and will be.Clin Cancer Res. 2013 Oct 1;19(19):5283-91. doi: 10.1158/1078-0432.CCR-13-2151. Clin Cancer Res. 2013. PMID: 24089441 Free PMC article. Review.
-
Construction, Expression, and Characterization of rSEA-EGF and In Vitro Evaluation of its Antitumor Activity Against Nasopharyngeal Cancer.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818762910. doi: 10.1177/1533033818762910. Technol Cancer Res Treat. 2018. PMID: 29551087 Free PMC article.
-
The T cell activating properties and antitumour activity of Staphylococcal Enterotoxin-like Q.Med Microbiol Immunol. 2019 Dec;208(6):781-792. doi: 10.1007/s00430-019-00614-9. Epub 2019 Jun 11. Med Microbiol Immunol. 2019. PMID: 31187242
References
-
- Chang AE, Aruga A, Cameron MJ et al. Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J Clin Oncol. 1997;15:796–807. - PubMed
-
- Chang AE, Li Q, Jiang G et al. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884–890. - PubMed
-
- Wang L, Chen B, Plautz GE. Adoptive immunotherapy of advanced tumors with CD62 L-selectinlow tumor-sensitized T lymphocytes following ex vivo hyperexpansion. J Immunol. 2002;169:3314–3320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

