The effect of glutamine infusion on the inflammatory response and HSP70 during human experimental endotoxaemia
- PMID: 19173710
- PMCID: PMC2688119
- DOI: 10.1186/cc7696
The effect of glutamine infusion on the inflammatory response and HSP70 during human experimental endotoxaemia
Abstract
Introduction: Glutamine supplementation has beneficial effects on morbidity and mortality in critically ill patients, possibly in part through an attenuation of the proinflammatory cytokine response and a stimulation of heat shock protein (HSP)70. We infused either alanine-glutamine or saline during endotoxin challenge and measured plasma cytokines and HSP70 protein expression.
Methods: This crossover study, conducted in eight healthy young men, was double-blind, randomized and placebo-controlled. It was performed on 2 trial days, separated by a 4-week washout period. The volunteers received an infusion of alanine-glutamine at a rate of 0.025 g/(kg body weight x hour) or saline for 10 hours. After 2 hours, an intravenous bolus of Escherichia coli endotoxin (0.3 ng/kg) was administered. Blood samples were collected hourly for the following 8 hours. HSP70 protein content in isolated blood mononuclear cells (BMNCs) was measured by Western blotting.
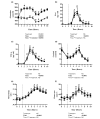
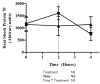
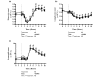
Results: Plasma glutamine increased during alanine-glutamine infusion. Endotoxin reduced plasma glutamine during both trials, but plasma glutamine levels remained above baseline with alanine-glutamine supplementation. Endotoxin injection was associated with alterations in white blood cell and differential counts, tumour necrosis factor-alpha, IL-6, temperature and heart rate, but glutamine affected neither the endotoxin-induced change in these variables nor the expression of HSP70 in BMNCs.
Conclusions: Endotoxin reduced plasma glutamine independently of alanine-glutamine infusion, but supplementation allows plasma levels to be maintained above baseline. Glutamine alters neither endotoxin-induced systemic inflammation nor early expression of HSP70 in BMNCs.
Trial registration: ClinicalTrials.gov ID: NCT 00780520.
Trial registration: ClinicalTrials.gov NCT00780520.
Figures
Similar articles
-
The effects of supra-normal protein C levels on markers of coagulation, fibrinolysis and inflammation in a human model of endotoxemia.Thromb Haemost. 2005 Dec;94(6):1148-55. doi: 10.1160/TH05-01-0059. Thromb Haemost. 2005. PMID: 16411386 Clinical Trial.
-
An in vitro model to consider the effect of 2 mM glutamine and KNK437 on endotoxin-stimulated release of heat shock protein 70 and inflammatory mediators.Nutrition. 2016 Mar;32(3):375-83. doi: 10.1016/j.nut.2015.09.007. Epub 2015 Sep 30. Nutrition. 2016. PMID: 26706024
-
The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells.Cell Stress Chaperones. 2015 Jan;20(1):85-93. doi: 10.1007/s12192-014-0528-1. Epub 2014 Jul 26. Cell Stress Chaperones. 2015. PMID: 25062931 Free PMC article.
-
Tissue factor pathway inhibitor dose-dependently inhibits coagulation activation without influencing the fibrinolytic and cytokine response during human endotoxemia.Blood. 2000 Feb 15;95(4):1124-9. Blood. 2000. PMID: 10666180 Clinical Trial.
-
Human experimental endotoxemia in modeling the pathophysiology, genomics, and therapeutics of innate immunity in complex cardiometabolic diseases.Arterioscler Thromb Vasc Biol. 2015 Mar;35(3):525-34. doi: 10.1161/ATVBAHA.114.304455. Epub 2014 Dec 30. Arterioscler Thromb Vasc Biol. 2015. PMID: 25550206 Free PMC article. Review.
Cited by
-
Daily Supplementation of L-Glutamine in Atrial Fibrillation Patients: The Effect on Heat Shock Proteins and Metabolites.Cells. 2020 Jul 20;9(7):1729. doi: 10.3390/cells9071729. Cells. 2020. PMID: 32698370 Free PMC article. Clinical Trial.
-
Characterization of differences in immune responses during bolus and continuous infusion endotoxin challenges using mathematical modelling.Exp Physiol. 2024 May;109(5):689-710. doi: 10.1113/EP091552. Epub 2024 Mar 11. Exp Physiol. 2024. PMID: 38466166 Free PMC article.
-
Prevalence of glutamine deficiency in ICU patients: a cross-sectional analytical study.Nutr J. 2016 Aug 2;15(1):73. doi: 10.1186/s12937-016-0188-3. Nutr J. 2016. PMID: 27485319 Free PMC article.
-
Year in review 2009: Critical Care--metabolism.Crit Care. 2010;14(6):238. doi: 10.1186/cc9256. Epub 2010 Nov 5. Crit Care. 2010. PMID: 21122170 Free PMC article. Review.
-
Intravenous glutamine decreases lung and distal organ injury in an experimental model of abdominal sepsis.Crit Care. 2009;13(3):R74. doi: 10.1186/cc7888. Epub 2009 May 19. Crit Care. 2009. PMID: 19454012 Free PMC article.
References
-
- Gamrin L, Essen P, Forsberg AM, Hultman E, Wernerman J. A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy-rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Crit Care Med. 1996;24:575–583. doi: 10.1097/00003246-199604000-00005. - DOI - PubMed
-
- Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48:297–309. - PubMed
-
- Dechelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coeffier M, Hecketsweiler B, Merle V, Mazerolles M, Samba D, Guillou YM, Petit J, Mansoor O, Colas G, Cohendy R, Barnoud D, Czernichow P, Bleichner G. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34:598–604. doi: 10.1097/01.CCM.0000201004.30750.D1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

