Highly efficient transduction of human plasmacytoid dendritic cells without phenotypic and functional maturation
- PMID: 19173717
- PMCID: PMC2657113
- DOI: 10.1186/1479-5876-7-10
Highly efficient transduction of human plasmacytoid dendritic cells without phenotypic and functional maturation
Abstract
Background: Gene modified dendritic cells (DC) are able to modulate DC functions and induce therapeutic immunity or tolerance in an antigen-specific manner. Among the different DC subsets, plasmacytoid DC (pDC) are well known for their ability to recognize and respond to a variety of viruses by secreting high levels of type I interferon.
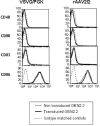
Methods: We analyzed here, the transduction efficiency of a pDC cell line, GEN2.2, and of pDC derived from CD34+ progenitors, using lentiviral vectors (LV) pseudotyped with different envelope glycoproteins such as the vesicular stomatitis virus envelope (VSVG), the gibbon ape leukaemia virus envelope (GaLV) or the feline endogenous virus envelope (RD114). At the same time, we evaluated transgene expression (E-GFP reporter gene) under the control of different promoters.
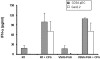
Results: We found that efficient gene transfer into pDC can be achieved with VSVG-pseudotyped lentiviral vectors (LV) under the control of phoshoglycerate kinase (PGK) and elongation factor-1 (EF1alpha) promoters (28% to 90% of E-GFP+ cells, respectively) in the absence of phenotypic and functional maturation. Surprisingly, promoters (desmin or synthetic C5-12) described as muscle-specific and which drive gene expression in single strand AAV vectors in gene therapy protocols were very highly active in pDC using VSVG-LV.
Conclusion: Taken together, our results indicate that LV vectors can serve to design pDC-based vaccines in humans, and they are also useful in vitro to evaluate the immunogenicity of the vector preparations, and the specificity and safety of given promoters used in gene therapy protocols.
Figures





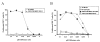
Similar articles
-
Efficient transduction of monocyte- and CD34+-derived Langerhans cells with lentiviral vectors in the absence of phenotypic and functional maturation.J Gene Med. 2006 Aug;8(8):951-61. doi: 10.1002/jgm.923. J Gene Med. 2006. PMID: 16741998
-
HIV-1-derived lentiviral vectors directly activate plasmacytoid dendritic cells, which in turn induce the maturation of myeloid dendritic cells.Hum Gene Ther. 2011 Feb;22(2):177-88. doi: 10.1089/hum.2010.085. Epub 2011 Jan 14. Hum Gene Ther. 2011. PMID: 20825284
-
Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates.Blood. 2002 Aug 1;100(3):823-32. doi: 10.1182/blood-2001-11-0042. Blood. 2002. PMID: 12130492
-
Efficient gene transfer to human peripheral blood monocyte-derived dendritic cells using human immunodeficiency virus type 1-based lentiviral vectors.Hum Gene Ther. 2000 Sep 1;11(13):1901-9. doi: 10.1089/10430340050129512. Hum Gene Ther. 2000. PMID: 10986562
-
HIV-derived vectors for gene therapy targeting dendritic cells.Adv Exp Med Biol. 2013;762:239-61. doi: 10.1007/978-1-4614-4433-6_9. Adv Exp Med Biol. 2013. PMID: 22975878 Review.
Cited by
-
Oncolytic measles virus induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cytotoxicity by human myeloid and plasmacytoid dendritic cells.Oncoimmunology. 2016 Nov 18;6(1):e1261240. doi: 10.1080/2162402X.2016.1261240. eCollection 2017. Oncoimmunology. 2016. PMID: 28197384 Free PMC article.
-
Current progress and challenges in HIV gene therapy.Future Virol. 2011 Nov 1;6(11):1319-1328. doi: 10.2217/fvl.11.113. Future Virol. 2011. PMID: 22754586 Free PMC article.
-
Interferon priming is essential for human CD34+ cell-derived plasmacytoid dendritic cell maturation and function.Nat Commun. 2018 Aug 30;9(1):3525. doi: 10.1038/s41467-018-05816-y. Nat Commun. 2018. PMID: 30166549 Free PMC article.
-
Regulatory and Exhausted T Cell Responses to AAV Capsid.Hum Gene Ther. 2017 Apr;28(4):338-349. doi: 10.1089/hum.2017.022. Hum Gene Ther. 2017. PMID: 28323492 Free PMC article.
-
Enhancement of CTLs induced by DCs loaded with ubiquitinated hepatitis B virus core antigen.World J Gastroenterol. 2012 Mar 28;18(12):1319-27. doi: 10.3748/wjg.v18.i12.1319. World J Gastroenterol. 2012. PMID: 22493545 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

