Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues
- PMID: 19173758
- PMCID: PMC3770294
- DOI: 10.1017/S1462399409000969
Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues
Abstract
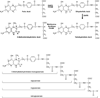
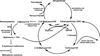
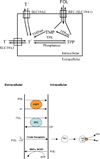
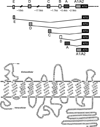
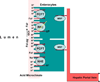
Members of the family of B9 vitamins are commonly known as folates. They are derived entirely from dietary sources and are key one-carbon donors required for de novo nucleotide and methionine synthesis. These highly hydrophilic molecules use several genetically distinct and functionally diverse transport systems to enter cells: the reduced folate carrier, the proton-coupled folate transporter and the folate receptors. Each plays a unique role in mediating folate transport across epithelia and into systemic tissues. The mechanism of intestinal folate absorption was recently uncovered, revealing the genetic basis for the autosomal recessive disorder hereditary folate malabsorption, which results from loss-of-function mutations in the proton-coupled folate transporter gene. It is therefore now possible to piece together how these folate transporters contribute, both individually and collectively, to folate homeostasis in humans. This review focuses on the physiological roles of the major folate transporters, with a brief consideration of their impact on the pharmacological activities of antifolates.
Figures

References
Reference List
-
- Stokstad ELR. Historical perspective on key advances in the biochemistry and physiology of folates. In: Picciano MF, E. Stokstad LR, editors. Folic Acid Metabolism in Health and Disease. New York: Wiley-Liss; 1990. pp. 1–21.
-
- Jacques PF, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. - PubMed
-
- Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403–456. - PubMed
-
- Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007;26:111–128. - PubMed
-
- Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004;56:1085–1097. - PubMed
9.0 Website
-
- For an in-depth up-to-date review of hereditary folate Malabsorption from the clinical and genetic perspectives. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=folate-mal.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

