Use of primary care electronic medical record database in drug efficacy research on cardiovascular outcomes: comparison of database and randomised controlled trial findings
- PMID: 19174434
- PMCID: PMC2769067
- DOI: 10.1136/bmj.b81
Use of primary care electronic medical record database in drug efficacy research on cardiovascular outcomes: comparison of database and randomised controlled trial findings
Abstract
Objectives: To determine whether observational studies that use an electronic medical record database can provide valid results of therapeutic effectiveness and to develop new methods to enhance validity.
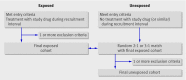
Design: Data from the UK general practice research database (GPRD) were used to replicate previously performed randomised controlled trials, to the extent that was feasible aside from randomisation. Studies Six published randomised controlled trials.
Main outcome measure: Cardiovascular outcomes analysed by hazard ratios calculated with standard biostatistical methods and a new analytical technique, prior event rate ratio (PERR) adjustment.
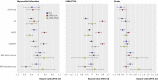
Results: In nine of 17 outcome comparisons, there were no significant differences between results of randomised controlled trials and database studies analysed using standard biostatistical methods or PERR analysis. In eight comparisons, Cox adjusted hazard ratios in the database differed significantly from the results of the randomised controlled trials, suggesting unmeasured confounding. In seven of these eight, PERR adjusted hazard ratios differed significantly from Cox adjusted hazard ratios, whereas in five they didn't differ significantly, and in three were more similar to the hazard ratio from the randomised controlled trial, yielding PERR results more similar to the randomised controlled trial than Cox (P<0.05).
Conclusions: Although observational studies using databases are subject to unmeasured confounding, our new analytical technique (PERR), applied here to cardiovascular outcomes, worked well to identify and reduce the effects of such confounding. These results suggest that electronic medical record databases can be useful to investigate therapeutic effectiveness.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Choosing between randomised and non-randomised studies: a systematic review. Health Technol Assess 1998;2:1-124. - PubMed
-
- MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS. A systematic review of comparisons of effect-sizes derived from randomized and non-randomised studies. Health Technol Assess 2000;4:1-154. - PubMed
-
- Benson K, Hartz AJ. A comparison of observational studies and randomized controlled trials. N Engl J Med 2000;342:1878-86. - PubMed
-
- Concato J, Horwitz RI. Beyond randomized versus observational studies. Lancet 2004;363:1660-1. - PubMed
-
- Sacks HS. Observational studies and randomized trials. N Engl J Med 2000;343:1195. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
