Functional expression of the olfactory signaling system in the kidney
- PMID: 19174512
- PMCID: PMC2644163
- DOI: 10.1073/pnas.0812859106
Functional expression of the olfactory signaling system in the kidney
Abstract
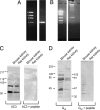
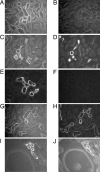
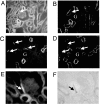
Olfactory-like chemosensory signaling occurs outside of the olfactory epithelium. We find that major components of olfaction, including olfactory receptors (ORs), olfactory-related adenylate cyclase (AC3) and the olfactory G protein (G(olf)), are expressed in the kidney. AC3 and G(olf) colocalize in renal tubules and in macula densa (MD) cells which modulate glomerular filtration rate (GFR). GFR is significantly reduced in AC3(-/-) mice, suggesting that AC3 participates in GFR regulation. Although tubuloglomerular feedback is normal in these animals, they exhibit significantly reduced plasma renin levels despite up-regulation of COX-2 expression and nNOS activity in the MD. Furthermore, at least one member of the renal repertoire of ORs is expressed in a MD cell line. Thus, key components of olfaction are expressed in the renal distal nephron and may play a sensory role in the MD to modulate both renin secretion and GFR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Buck L, Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. - PubMed
-
- Wong ST, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. - PubMed
-
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. - PubMed
-
- Spehr M, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. - PubMed
-
- Livera G, et al. Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol Endocrinol. 2005;19:1277–1290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

