Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines
- PMID: 19174557
- PMCID: PMC2692390
- DOI: 10.1158/1535-7163.MCT-08-0854
Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines
Abstract
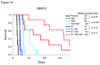
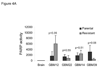
Resistance to temozolomide and radiotherapy is a major problem for patients with glioblastoma but may be overcome using the poly(ADP-ribose) polymerase inhibitor ABT-888. Using two primary glioblastoma xenografts, the efficacy of ABT-888 combined with radiotherapy and/or temozolomide was evaluated. Treatment with ABT-888 combined with temozolomide resulted in significant survival prolongation (GBM12: 55.1%, P = 0.005; GBM22: 54.4%, P = 0.043). ABT-888 had no effect with radiotherapy alone but significantly enhanced survival in GBM12 when combined with concurrent radiotherapy/temozolomide. With multicycle therapy, ABT-888 further extended the survival benefit of temozolomide in the inherently sensitive GBM12 and GBM22 xenograft lines. However, after in vivo selection for temozolomide resistance, the derivative GBM12TMZ and GBM22TMZ lines were no longer sensitized by ABT-888 in combination with temozolomide, and a similar lack of efficacy was observed in two other temozolomide-resistant tumor lines. Thus, the sensitizing effects of ABT-888 were limited to tumor lines that have not been previously exposed to temozolomide, and these results suggest that patients with newly diagnosed glioblastoma may be more likely to respond to combined temozolomide/poly(ADP-ribose) polymerase inhibitor therapy than patients with recurrent disease.
Figures









References
-
- Stupp R, Dietrich PY, Ostermann Kraljevic S. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002 Mar 1;20(5):1375–1382. [see comment] - PubMed
-
- Stupp R, Mason WP, van den Bent MJ. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005 March 10;352(10):987–996. 2005. - PubMed
-
- Hegi ME, Diserens A-C, Gorlia T. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med. 2005 March 10;352(10):997–1003. 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

