Structural insights into TDP-43 in nucleic-acid binding and domain interactions
- PMID: 19174564
- PMCID: PMC2665213
- DOI: 10.1093/nar/gkp013
Structural insights into TDP-43 in nucleic-acid binding and domain interactions
Abstract
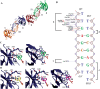
TDP-43 is a pathogenic protein: its normal function in binding to UG-rich RNA is related to cystic fibrosis, and inclusion of its C-terminal fragments in brain cells is directly linked to frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). Here we report the 1.65 A crystal structure of the C-terminal RRM2 domain of TDP-43 in complex with a single-stranded DNA. We show that TDP-43 is a dimeric protein with two RRM domains, both involved in DNA and RNA binding. The crystal structure reveals the basis of TDP-43's TG/UG preference in nucleic acids binding. It also reveals that RRM2 domain has an atypical RRM-fold with an additional beta-strand involved in making protein-protein interactions. This self association of RRM2 domains produced thermal-stable RRM2 assemblies with a melting point greater than 85 degrees C as monitored by circular dichroism at physiological conditions. These studies thus characterize the recognition between TDP-43 and nucleic acids and the mode of RRM2 self association, and provide molecular models for understanding the role of TDP-43 in cystic fibrosis and the neurodegenerative diseases related to TDP-43 proteinopathy.
Figures

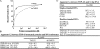
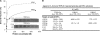
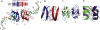

References
-
- Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 2008;13:867–878. - PubMed
-
- Talbot K, Ansorge O. Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: common pathways in neurodegenerative disease. Human Mol. Genet. 2006;15:182–187. - PubMed
-
- Kwong LK, Uryu K, Trojanowski JQ, Lee VM-Y. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals. 2008;16:41–51. - PubMed
-
- Abhyankar MM, Urekar C, Reddi PP. A novel CpG-free vertebrate insulator silences the testis-specific SP-10 gene in somatic tissues- role for TDP-43 in insulator function. J. Biol. Chem. 2007;282:36143–36154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

