Identification of modulated genes by three classes of chemopreventive agents at preneoplastic stages in a p53-null mouse mammary tumor model
- PMID: 19174580
- PMCID: PMC3023963
- DOI: 10.1158/1940-6207.CAPR-08-0104
Identification of modulated genes by three classes of chemopreventive agents at preneoplastic stages in a p53-null mouse mammary tumor model
Abstract
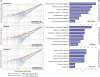
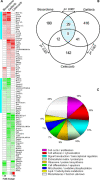
Genetically engineered mouse cancer models are among the most useful tools for testing the in vivo effectiveness of the various chemopreventive approaches. The p53-null mouse model of mammary carcinogenesis was previously characterized by us at the cellular, molecular, and pathologic levels. In a companion article, Medina et al. analyzed the efficacy of bexarotene, gefitinib, and celecoxib as chemopreventive agents in the same model. Here we report the global gene expression effects on mammary epithelium of such compounds, analyzing the data in light of their effectiveness as chemopreventive agents. SAGE was used to profile the transcriptome of p53-null mammary epithelium obtained from mice treated with each compound versus controls. This information was also compared with SAGE data from p53-null mouse mammary tumors. Gene expression changes induced by the chemopreventive treatments revealed a common core of 87 affected genes across treatments (P < 0.05). The effective compounds, bexarotene and gefitinib, may exert their chemopreventive activity, at least in part, by affecting a set of 34 genes related to specific cellular pathways. The gene expression signature revealed various genes previously described to be associated with breast cancer, such as the activator protein-1 complex member Fos-like antigen 2 (Fosl2), early growth response 1 (Egr1), gelsolin (Gsn), and tumor protein translationally controlled 1 (Tpt1), among others. The concerted modulation of many of these transcripts before malignant transformation seems to be conducive to predominantly decrease cell proliferation. This study has revealed candidate key pathways that can be experimentally tested in the same model system and may constitute novel targets for future translational research.
Figures

References
-
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. - PubMed
-
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenospausal women: results form the MORE randomized trial. Multiple Outcomes of Raloxifene evaluation. JAMA. 1999;281:2189–2197. - PubMed
-
- Shen Q, Brown PH. Transgenic mouse models for the prevention of breast cancer. Mutat Res. 2005;576:93–110. - PubMed
-
- Jerry DJ, Kittrell FS, Kuperwasser C, Laucirica R, Dickinson ES, Bonilla PJ, Butel JS, Medina D. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–8. - PubMed
-
- Medina D, Kittrell FS, Shepard A, Stephens LC, Jiang C, Lu J, Allred DC, Mccarthy M, Ullrich RL. Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J. 2002;16:881–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

