Strategies for innovation in multicomponent reaction design
- PMID: 19175315
- PMCID: PMC2765544
- DOI: 10.1021/ar800214s
Strategies for innovation in multicomponent reaction design
Abstract
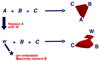
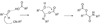
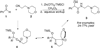
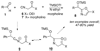
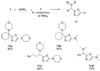
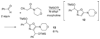
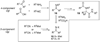
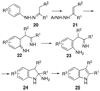
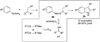
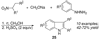
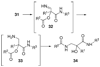
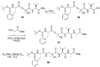
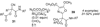
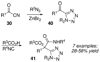
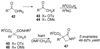
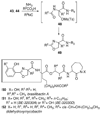
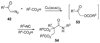
By generating structural complexity in a single step from three or more reactants, multicomponent reactions (MCRs) make it possible to synthesize target compounds with greater efficiency and atom economy. The history of such reactions can be traced to the mid-19th century when Strecker first produced alpha-aminonitriles from the condensation of aldehydes with ammonia and hydrogen cyanide. Recently, academic chemists have renewed their interest in MCRs. In part, the pharmaceutical industry has fueled this resurgence because of the growing need to assemble libraries of structurally complex substances for evaluation as lead compounds in drug discovery and development programs. The application of MCRs to that increasingly important objective remains limited by the relatively small number of such reactions that can be broadly applied to prepare biologically relevant or natural-product-like molecular frameworks. We were interested in applying logic-based approaches, such as our single reactant replacement (SRR) approach, as a way both to improve known MCRs and to design new multiple-component routes to bioactive structures. This Account provides several examples that illustrate the use of SRR with known MCRs as starting points for synthetic innovation in this area. As part of our working hypothesis, we initially explored strategies for engineering improvements into known MCRs, either by increasing the dimensionality--that is, changing an n-component to an (n + 1)-component reaction--or broadening the scope of useful input structures, or both. By exhaustively applying retrosynthetic analysis to the cognate MCR to identify and exploit alternative entry points into the overall reaction manifold, we have devised several such re-engineered MCRs. Serendipitous findings have also augmented the yield of useful developments from our logic-inspired approach. In some cases, we have identified surprising links between different compound families that provide useful new entry points for chemical library synthesis. In other cases, the same re-engineering logic made it possible (sometimes in unexpected ways) to transform certain nonelementary two-component reactions into higher order MCRs. While logic may also inspire the search for new MCRs, the design process requires added chemical creativity, which cannot be reduced to a simple formula. The long-term goal of our research is to expand the useful repertoire of such reactions, which are important as complexity-generating tools in both combinatorial and diversity-oriented synthesis.
Figures


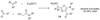

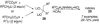

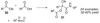
References
-
- Ugi I, Dömling A, Hörl W. Multicomponent Reactions in Organic Chemistry. Endeavour. 1994;18:115–112.
- Armstrong RM, Combs AP, Tempest PA, Brown SD, Keating TA. Multiple-Component Condensation Strategies for Combinatorial Library Synthesis. Acc. Chem. Res. 1996;29:123–131.
-
- Bienaymé H, Hulme C, Oddon G, Schmitt P. Maximizing Synthetic Efficiency: Multicomponent Transformations Lead the Way. Chem. Eur. J. 2000;6:3321–3329. - PubMed
-
- Hulme C, Gore V. Multi-component Reactions: Emerging Chemistry in Drug Discovery. From Xylocaine to Crixivan. Curr. Medicinal Chem. 2003;10:51–80. - PubMed
-
- Dömling A. Recent Developments in Isocyanide Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006;106:17–89. - PubMed
- Dömling A, Ugi I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000;39:3169–3210. - PubMed
- Doemling A. The Discovery of New Multicomponent Reactions. Curr. Opin. Chem. Biol. 2000;4:318–323. - PubMed
-
- Ramon DJ, Yus M. Asymmetric Multicomponent Reactions: The New Frontier. Angew. Chem. Int. Ed. 2005;44:1602–1634. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

