Tobacco-induced alterations to Porphyromonas gingivalis-host interactions
- PMID: 19175666
- PMCID: PMC2713102
- DOI: 10.1111/j.1462-2920.2008.01852.x
Tobacco-induced alterations to Porphyromonas gingivalis-host interactions
Abstract
Smokers are more susceptible than non-smokers to persistent infection by Porphyromonas gingivalis, a causative agent of periodontitis. Patients who smoke exhibit increased susceptibility to periodontitis and are more likely to display severe disease and be refractory to treatment. Paradoxically, smokers demonstrate reduced clinical inflammation. We show that P. gingivalis cells exposed to cigarette smoke extract (CSE) induce a lower proinflammatory response (tumour necrosis factor-alpha, interleukin-6, interleukin-12 p40) from monocytes and peripheral blood mononuclear cells than do unexposed bacteria. This effect is reversed when CSE-exposed bacteria are subcultured in fresh medium without CSE. Using microarrays representative of the P. gingivalis genome, CSE-exposure resulted in differential regulation of 6.8% of P. gingivalis genes, including detoxification and oxidative stress-related genes; DNA repair genes; and multiple genes related to P. gingivalis virulence, including genes in the major fimbrial and capsular operons. Exposure to CSE also altered the expression of outer membrane proteins, most notably by inducing the virulence factors RagA and RagB, and a putative lipoprotein cotranscribed with the minor fimbrial antigen. Therefore, CSE represents an environmental stress to which P. gingivalis adapts by altering gene expression and outer membrane proteins. These changes may explain, in part, the altered virulence and host-pathogen interactions that have been documented in vivo in smokers with periodontal disease.
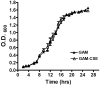
Figures









References
-
- Araco A, Gravante G, Sorge R, Araco F, Delogu D, Cervelli V. Wound infections in aesthetic abdominoplasties: the role of smoking. Plast Reconstr Surg. 2008;121:305e–310e. - PubMed
-
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. - PubMed
-
- Barbour SE, Nakashima K, Zhang JB, Tangada S, Hahn CL, Schenkein HA, Tew JG. Tobacco and smoking: environmental factors that modify the host response (immune system) and have an impact on periodontal health. Crit Rev Oral Biol Med. 1997;8:437–460. - PubMed
-
- Bernard D. In: Molecular Mechanisms of Tobacco-Induced Diseases. Wang XL, Scott DA, editors. Nova Science; 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

