Plasma and memory B-cell kinetics in infants following a primary schedule of CRM 197-conjugated serogroup C meningococcal polysaccharide vaccine
- PMID: 19175802
- PMCID: PMC2678189
- DOI: 10.1111/j.1365-2567.2008.02934.x
Plasma and memory B-cell kinetics in infants following a primary schedule of CRM 197-conjugated serogroup C meningococcal polysaccharide vaccine
Erratum in
- Immunology. 2011 Apr;132(4):589
Abstract
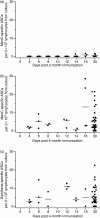
The induction of persistent protective levels of pathogen-specific antibody is an important goal of immunization against childhood infections. However, antibody persistence is poor after immunization in infancy versus later in life. Serogroup C meningococci (MenC) are an important cause of bacteraemia and meningitis in children. The use of protein-polysaccharide conjugate vaccines against MenC has been associated with a significant decline in the incidence of invasive disease. However, vaccine effectiveness is negligible by more than 1 year after a three-dose priming series in infancy and corresponds to a rapid decline in antibody following an initial immune response. The cellular mechanisms underlying the generation of persistent antibody in this age group are unclear. An essential prelude to larger studies of peripheral blood B cells is an understanding of B-cell kinetics following immunization. We measured MenC- and diphtheria-specific plasma and memory B-cell kinetics in infants receiving a CRM(197) (cross-reactive material; mutant diphtheria toxoid)-conjugated MenC vaccine at 2, 3 and 4 months of age. Plasma cell responses were more delayed after the first dose when compared with the rapid appearance of plasma cells after the third dose. Memory B cells were detectable at all time-points following the third dose as opposed to the low frequency seen following a first dose. This study provides data on B-cell kinetics following a primary schedule of immunization in young infants upon which to base further studies of the underlying cellular mechanism of humoral immunity.
Figures


Similar articles
-
Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial.BMJ. 2015 Apr 1;350:h1554. doi: 10.1136/bmj.h1554. BMJ. 2015. PMID: 25832102 Free PMC article. Clinical Trial.
-
The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response.J Immunol. 2008 Feb 15;180(4):2165-73. doi: 10.4049/jimmunol.180.4.2165. J Immunol. 2008. PMID: 18250423
-
CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells.Blood. 2006 Oct 15;108(8):2642-7. doi: 10.1182/blood-2006-01-009282. Epub 2006 May 4. Blood. 2006. PMID: 16675705 Clinical Trial.
-
Update on the use of meningococcal serogroup C CRM₁₉₇-conjugate vaccine (Meningitec) against meningitis.Expert Rev Vaccines. 2016;15(1):9-29. doi: 10.1586/14760584.2016.1115726. Epub 2015 Nov 26. Expert Rev Vaccines. 2016. PMID: 26560735 Review.
-
Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV).Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S97-S108. doi: 10.1097/INF.0b013e318199f61b. Pediatr Infect Dis J. 2009. PMID: 19325452 Review.
Cited by
-
Exploring the variables influencing the immune response of traditional and innovative glycoconjugate vaccines.Front Mol Biosci. 2023 May 16;10:1201693. doi: 10.3389/fmolb.2023.1201693. eCollection 2023. Front Mol Biosci. 2023. PMID: 37261327 Free PMC article. Review.
-
Circulating human antibody-secreting cells during vaccinations and respiratory viral infections are characterized by high specificity and lack of bystander effect.J Immunol. 2011 May 1;186(9):5514-21. doi: 10.4049/jimmunol.1002932. Epub 2011 Mar 25. J Immunol. 2011. PMID: 21441455 Free PMC article.
-
Production of PfEMP1-Specific Human Monoclonal Antibodies from Naturally Immune Individuals.Methods Mol Biol. 2022;2470:407-421. doi: 10.1007/978-1-0716-2189-9_30. Methods Mol Biol. 2022. PMID: 35881362
-
Antibodies in lymphocyte supernatants can distinguish between neutralising antibodies induced by RSV vaccination and pre-existing antibodies induced by natural infection.Vaccine. 2018 Nov 12;36(46):6988-6994. doi: 10.1016/j.vaccine.2018.09.070. Epub 2018 Oct 11. Vaccine. 2018. PMID: 30318168 Free PMC article. Clinical Trial.
-
Long-term evolution of antigen repertoires among carried meningococci.Proc Biol Sci. 2010 Jun 7;277(1688):1635-41. doi: 10.1098/rspb.2009.2033. Epub 2010 Feb 3. Proc Biol Sci. 2010. PMID: 20129981 Free PMC article.
References
-
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. - PubMed
-
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. - PubMed
-
- Kelly DF, Pollard AJ, Moxon ER. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. Jama. 2005;294:3019–23. - PubMed
-
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(12 Suppl):S274–9. - PubMed
-
- MacLennan JM, Shackley F, Heath PT, et al. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. Jama. 2000;283:2795–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

