Expression of ovarian tumour suppressor OPCML in the female CD-1 mouse reproductive tract
- PMID: 19176311
- PMCID: PMC2665191
- DOI: 10.1530/REP-08-0511
Expression of ovarian tumour suppressor OPCML in the female CD-1 mouse reproductive tract
Abstract
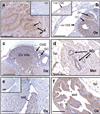
Opioid binding protein/cell adhesion molecule-like gene (OPCML) is frequently inactivated in epithelial ovarian cancer, but the role of this membrane protein in normal reproductive function is unclear. The ovarian surface epithelium (OSE) is thought to be the cell of origin of most epithelial ovarian cancers, some of which arise after transformation of OSE cells lining ovarian inclusion cysts, formed during ovulation. We used immunohistochemistry, immunoblotting and quantitative RT-PCR (qRT-PCR) to investigate OPCML expression in the uteri and ovaries of cycling 3-month CD-1 mice, as well as in ovaries from older mice containing inclusion cysts derived from rete ovarii tubules. Immunoblotting showed OPCML bands in uterine, but not whole ovarian or muscle extracts. Strong OPCML immunoreactivity was observed in oviduct, rete ovarii and uterus, whereas in ovary more immunoreactivity was seen in granulosa cells than OSE. No staining was observed in OSE around ovulation sites, where OSE cells divide to cover the site. OPCML immunoreactivity was also weaker in more dysplastic cells lining large ovarian inclusion cysts, compared with normal rete ovarii. No significant changes in Opcml mRNA expression were observed in whole ovarian and uterine extracts at different stages of the cycle. We conclude that murine OPCML is more consistently expressed in cells lining the uterus, oviduct and rete ovarii than in ovary and is not expressed in OSE associated with ovulation sites. This observation supports the hypothesis that a proportion of epithelial ovarian cancers arise from ductal cells and other epithelia of the secondary Mullerian system, rather than the OSE.
Figures


References
-
- Blaustein A. Surface (germinal) epithelium and related ovarian neoplasms. Pathology Annual. 1981;16:247–94. - PubMed
-
- Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. Journal of Clinical Oncology. 2007;25:3985–90. - PubMed
-
- Chen H, Ye F, Zhang J, Lu W, Cheng Q, Xie X. Loss of OPCML expression and the correlation with CpG island methylation and LOH in ovarian serous carcinoma. European Journal of Gynaecological Oncology. 2007;28:464–7. - PubMed
-
- Clow OL, Hurst PR, Fleming JS. Changes in the mouse ovarian surface epithelium with age and ovulation number. Molecular and Cellular Endocrinology. 2002;191:105–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

